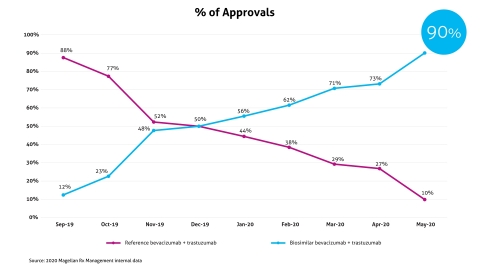
Magellan Rx Medical Pharmacy Solution Impact: 90% of New Authorizations for Oncology Biosimilars
Magellan Rx Management, a division of Magellan Health, Inc. (NASDAQ: MGLN), today released preliminary results from its oncology biosimilar medical pharmacy solution that targets new-to-market oncology therapeutic biosimilars. These results further demonstrate Magellan Rx Management’s innovative capabilities and expertise in the specialty drug market. As of May 2020, health plan customers who were quick to partner with Magellan Rx have achieved a
Magellan Rx Management (Graphic: Business Wire)
“These results, less than one year after implementation, highlight Magellan Rx’s passion and commitment to developing forward-thinking, industry-leading solutions to manage one of the most complex and evolving areas of healthcare—medical pharmacy drug spend,” said Steve Cutts, PharmD, senior vice president and general manager, specialty, Magellan Rx Management. “Given our 17-year history in medical benefit management, combined with innovative comprehensive oncology solutions and our prior success in advancing biosimilar utilization, we are uniquely qualified to support our health plan customers to deliver true savings while maintaining or expanding member access to clinically-effective, lower-cost treatments.”
Health plan customers implemented this program throughout the fall and winter of 2019, shortly after the July 2019 arrival of oncology biosimilars on the market, with several more opting into this innovative program through early 2020. The
To learn more about how Magellan Rx Management’s solution can increase the use of less-expensive biosimilars, view this poster that was presented at the Academy of Managed Care Pharmacy Annual Spring Conference in April 2020. For more information on the oncology biosimilar landscape, watch this webisode of MRx Events @ Home featuring one of our in-house specialty experts, Rebecca Borgert, PharmD, BCOP, senior director, clinical strategy and programs.
- Amgen Inc. (2019) Amgen And Allergan's MVASI™ (bevacizumab-awwb) And KANJINTI™ (trastuzumab-anns) Now Available In The United States [Press release]. 18 July. Available at: https://www.amgen.com/media/news-releases/2019/07/amgen-and-allergans-mvasi-bevacizumabawwb-and-kanjinti-trastuzumabanns-now-available-in-the-united-states/ (Accessed: 26 August 2020).
- 2019 Magellan Rx Management Medical Pharmacy Trend Report™, ©2020.
About Magellan Rx Management: Magellan Rx Management, a division of Magellan Health, Inc., is a next-generation pharmacy organization that is delivering meaningful solutions to the people we serve. As pioneers in specialty drug management, industry leaders in Medicaid pharmacy programs and disruptors in pharmacy benefit management, we partner with our customers and members to deliver a best-in-class healthcare experience.
About Magellan Health: Magellan Health, Inc. is a leader in managing the fastest growing, most complex areas of health, including special populations, complete pharmacy benefits and other specialty areas of healthcare. Magellan supports innovative ways of accessing better health through technology, while remaining focused on the critical personal relationships that are necessary to achieve a healthy, vibrant life. Magellan's customers include health plans and other managed care organizations, employers, labor unions, various military and governmental agencies and third-party administrators. For more information, visit MagellanHealth.com.
(MGLN-GEN)
View source version on businesswire.com: https://www.businesswire.com/news/home/20210201005224/en/







