MEI Pharma and Kyowa Kirin Announce New Clinical Data on Zandelisib at American Society of Clinical Oncology Annual Meeting 2021
On May 19, 2021, MEI Pharma announced promising data for zandelisib, a PI3Kδ inhibitor, aimed at treating B-cell malignancies. Key findings include an 87% overall response rate in relapsed or refractory follicular lymphoma patients, with 93% in non-POD24 patients. The Phase 3 COASTAL study, comparing zandelisib plus rituximab to standard chemotherapy in indolent non-Hodgkin's lymphoma, is set to enroll 534 patients. Topline results from the ongoing Phase 2 TIDAL study are expected later this year, reinforcing the commitment to zandelisib's development as a potential best-in-class therapy.
- 87% overall response rate in relapsed or refractory follicular lymphoma patients.
- 93% response rate in non-POD24 patients.
- Zandelisib demonstrated high tolerability with only 8% discontinuation due to adverse events.
- Phase 3 COASTAL study aims to enroll 534 patients, indicating strong commitment to zandelisib's clinical development.
- None.
Insights
Analyzing...
SAN DIEGO and TOKYO, May 19, 2021 /PRNewswire/ -- MEI Pharma, Inc. (NASDAQ: MEIP), a late-stage pharmaceutical company focused on advancing new therapies for cancer, and Kyowa Kirin Co., Ltd. (Kyowa Kirin, TSE: 4151), a global specialty pharmaceutical company that utilizes the latest biotechnology to discover and deliver novel medicines, today announced new data for zandelisib, an investigational phosphatidylinositol 3-kinase delta ("PI3Kδ") inhibitor in clinical development for the treatment of B-cell malignancies. The research and the design of a Phase 3 study will be presented in three posters at the American Society of Clinical Oncology (ASCO) Annual Meeting 2021 being held virtually June 4-8, 2021.
"We are encouraged by the zandelisib data being shared at this year's ASCO Annual Meeting, specifically the data that showed zandelisib activity across differing patient groups, including POD24 – a group that typically has a poor prognosis and would generally be expected to meet the inclusion criteria of our Phase 3 COASTAL study. Additionally, the data reporting on the combination of zandelisib and zanubrutinib, demonstrated the potential to induce robust and durable responses against various B-cell malignancies with a combination that is generally well-tolerated," said Richard Ghalie, M.D., chief medical officer at MEI Pharma. "With topline data from the Phase 2 TIDAL study on track to be reported by the end of this year, we remain committed, in collaboration with our global partner Kyowa Kirin, to the zandelisib development program and its potential to deliver a best-in-class treatment option to patients with B-cell malignancies as a monotherapy or in combination with other therapies."
The presentations will present updated data from a Phase 1b study of zandelisib +/- rituximab that show the compound continues to be generally well tolerated with an
Additionally, the presentations will include new data from the Phase 1b trial exploring zandelisib in combination with zanubrutinib (marketed as BRUKINSA®), an inhibitor of Bruton's tyrosine kinase ("BTK") developed by BeiGene, Ltd. ("BeiGene"), in patients with (r/r) B-cell malignancies. In this study, the combination of zandelisib and zanubrutinib was generally well tolerated in the 20 patients enrolled in the safety evaluation cohort. The combination administered on an optimized dosing regimen did not result in additive toxicity to each agent alone. Further,
The trial design for the Phase 3 COASTAL study evaluating zandelisib in combination with rituximab in patients with r/r indolent non-Hodgkin's lymphoma (iNHL), will also be highlighted in a poster presentation. The COASTAL study is intended to act as the required confirmatory study for the potential U.S. accelerated approval of zandelisib in patients with r/r FL or marginal zone lymphoma (MZL) who have received one or more prior lines of treatment and is also intended to support FDA approval for additional indications and regulatory marketing applications globally.
"Data from the early Phase 1 trials indicate that zandelisib displays high selectivity for the phosphatidylinositol 3-kinase delta (PI3K) isoform as well as durability, with PI3K playing a key role in the proliferation and survival of hematologic cancers," said Yoshifumi Torii, Ph.D., Executive Officer, Vice President, Head of R&D Division of Kyowa Kirin. "Zandelisib has distinct pharmaceutical properties and we look forward to continuing the development of the compound, in partnership with MEI Pharma, with the hope that more data will add to the understanding of zandelisib and potentially yield another option for patients with B-cell malignancies."
Details on the three posters are included below.
Updated Clinical Data from the Phase 1b Study Evaluating Zandelisib in Patients with Relapsed or Refractory Follicular Lymphoma
- Poster title: Efficacy and Safety of the PI3Kδ Inhibitor Zandelisib (ME-401) on an Intermittent Schedule (IS) in Patients with Relapsed/Refractory Follicular Lymphoma (FL) with Progression of Disease within 24 Months of First-Line Chemoimmunotherapy (POD24)
- Available for on-demand viewing online: Beginning on June 4, 2021 at 6:00 a.m. PT here
- Poster will also be available for download via the MEI Pharma website
John Pagel, M.D., Ph.D., lead author on the poster, study investigator, and Chief of Hematologic Malignancies, Swedish Cancer Center, commented: "The group of relapsed or refractory follicular lymphoma patients that experience progression of disease within 24 months of first line chemoimmunotherapy have a poorer long-term prognosis compared to the patients with follicular lymphoma who relapse later than 24 months and represent an area of high need for new treatment options. The positive results reported today indicate the potential for zandelisib to provide a new treatment option for high-risk patients with relapsed or refractory follicular lymphoma."
Study Details:
The ongoing Phase 1b clinical study is a multi-arm, open-label, dose optimization trial evaluating zandelisib as a monotherapy and in combination with other therapies in patients with r/r B-cell malignancies. The data reported today is for patients receiving zandelisib administered on the intermittent schedule: once daily at 60 mg for two 28-day cycles and then on an intermittent schedule of once daily dosing for the first 7 days of each subsequent 28-day cycle (i.e. the intermittent schedule or IS). A total of 37 r/r FL patients have been treated with zandelisib on the intermittent schedule, as a monotherapy or in combination with rituximab. Of the 37 r/r FL patients, 22 were POD24. POD24 is a robust predictor in FL of reduced overall survival.
The overall response rate in 37 patients with r/r FL was
Overall Response Rate
All (N = 37) | POD24 (n = 22) | Non-POD24 (n = 15) | |
ORR, n (%)* | 32 (87) | 18 (82) | 14 (93) |
Monotherapy, n/N (%) Combination with rituximab, n/N (%) | 14/18 (78) 18/19 (95) | 8/11 (73) 10/11 (91) | 6/7 (86) 8/8 (100) |
Prior lines of therapy, n/N (%) 1 line of prior therapy ≥ 2 lines of prior therapy |
14/16 (88) 18/21 (86) |
5/7 (71) 13/15 (87) |
9/9 (100) 5/6 (83) |
CR rate, n (%) | 10 (27) | 4 (18) | 6 (40) |
* Imaging scans were obtained after 2 and 6 cycles, and then every 6 cycles. Response was reported based on Lugano criteria.
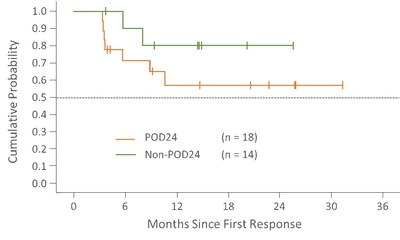
Duration of Response
Median duration of response in POD24 and non-POD24 patients has not yet been reached. Median duration follow-up is 15.8 months (range: 5.6-33.1) in POD24 patients and 17 months (range: 1.2-28.6) in non-POD24.
Follow-up = Time from first response to treatment discontinuation date or data cutoff of 10DEC2020 for ongoing patients
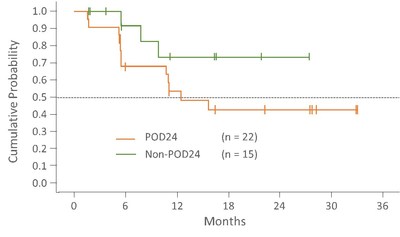
Progression Free Survival
Progression free survival in POD24 patients is 12.5 months and in the non-POD24 group the median is not yet reached. Median follow-up time in the POD24 and non-POD24 groups is 19.4 (range 1.8-36.5) and 18.2 (range: 3.0-30.4) months, respectively.
Follow-up = Time from first day on study to treatment discontinuation or data cutoff of 10DEC2020
Adverse Events
Zandelisib was generally well-tolerated. No difference in adverse events was observed between POD24 and non-POD24 groups. The discontinuation rate due to any treatment emergent adverse event was
Grade ≥3 Adverse Events in ≥2 Patients | N = 37 N (%) |
Neutropenia | 6 (16) |
ALT/AST increased | 3 (8) |
Rash | 3 (8) |
Diarrhea | 2 (5) |
Colitis | 2 (5) |
Hypokalemia | 2 (5) |
Hyponatremia | 2 (5) |
Coronavirus infection | 2 (5) |
Phase 1b Study Evaluating Zandelisib in Combination with Zanubrutinib in Patients with B-cell Malignancies
- Poster title: Initial Results of the Combination of PI3Kδ Inhibitor Zandelisib (ME-401) and the BTK Inhibitor Zanubrutinib in Patients (pts) with r/r B-cell Malignancies
- Available for on-demand viewing online: Beginning on June 4, 2021 at 6:00 a.m. PT here.
- Poster will also be available for download via the MEI Pharma website
Jacob Soumerai, M.D., lead author on the poster, study investigator and Assistant Professor at Harvard Medical School and Massachusetts General Hospital Cancer Center, commented: "The data reported today for zandelisib in combination with zanubrutinib are encouraging, both in terms of preliminary safety and efficacy, and support the expansion of the evaluation of this orally administered combination in various B-cell malignancies."
Study Details
The ongoing Phase 1b clinical study is a multi-arm, open-label, dose optimization trial evaluating zandelisib as a monotherapy and in combination with other therapies in patients with r/r B-cell malignancies. The data reported today is for 20 patients receiving zandelisib in combination with zanubrutinib. Two treatment dosing regimens were explored: Group A received zandelisib 60 mg, oral, daily continuously for 8 weeks followed by days 1-7 of each subsequent 28-day cycle, and zanubrutinib (marketed as BRUKINSA® by BeiGene, Ltd.), 160 mg oral, twice daily; Group B received zandelisib 60 mg, oral, daily on days 1-7 of each 28-day cycle starting Cycle 1 and zanubrutinib 80 mg, oral, twice daily. Group A enrolled a total of 7 patients: 1 FL, 3 CLL, 1 MZL, 1 mantle cell lymphoma (MCL), and 1 diffuse large B-cell lymphoma/high grade B-cell lymphoma (DLBCL/HGBCL). Group B enrolled a total of 13 patients: 7 FL, 2 CLL, 1 MZL, and 3 DLBCL/HGBCL. Treatment was continued until disease progression, intolerance or withdrawal of consent.
Overall Response Rates
The overall response rate in all evaluable patients with r/r indolent B-cell malignancies and CLL was
Evaluable n = 18 | FL | CLL/SLL | MZL | MCL | DLBCL/HGBCL |
ORR*, n (%) | 8 (100) | 5 (100) | 2 (100) | 1 (100) | 0 |
Group A | 1 (100) | 3 (100) | 1 (100) | 1 (100) | 0 |
Group B | 7 (100) | 2 (100) | 1 (100) | 0 | 0 |
*CR/CRi in 2/8 FL (
Treatment Emergent Adverse Events
The combination of zandelisib 60 mg oral, daily on days 1-7 of each 28-day cycle starting Cycle 1 and zanubrutinib 80 mg, oral, twice daily was well tolerated across the various B-cell malignancies in the completed part of the study. The combination administered on the optimized, Group B, dosing regimen did not result in additive toxicity to each agent alone. One of the two patients with Grade 3 AST/ALT increases in Group B was successfully retreated and continued therapy.
Group A (n = 7) | Group B (n = 13) | |||
Adverse Event, n (%) | All Grades | Grade ≥ 3 | All Grades | Grade ≥ 3 |
Neutropenia | 4 (57) | *3 (43) | 6 (46) | 3 (23) |
ALT increased | 2 (29) | 2 (29) | 2 (15) | **2 (15) |
AST increased | 2 (29) | 2 (29) | 3 (23) | **2 (15) |
Anemia | 1 (14) | *1 (14) | 2 (15) | 1 (8) |
Hyperkalemia | 2 (29) | 0 | 2 (15) | 1 (8) |
Thrombocytopenia | 4 (57) | *2 (29) | 3 (23) | 1 (8) |
Pleural Effusion | 2 (29) | *1 (14) | 0 | 0 |
Rash | 1 (14) | 1 (14) | 2 (15) | 0 |
Appendicitis | 0 | 0 | 1 (8) | 1 (8) |
Ascites | 1 (14) | *1 (14) | 0 | 0 |
CMV colitis | 1 (14) | 1 (14) | 0 | 0 |
Fatigue | 4 (57) | 1 (14) | 2 (15) | 0 |
Pneumonia | 2 (29) | *1 (14) | 0 | 0 |
Tumor lysis syndrome | 0 | 0 | 1 (8) | 1 (8) |
Diarrhea | 3 (43) | 0 | 3 (23) | 0 |
Atrial fibrillation | 1 (14) | 0 | 0 | 0 |
* Group A: 1 DLBCL patient experienced Grade 3 AE on Day 0 attributed to prior therapy and discontinued treatment on Day 17, then progressed on Day 23 and died with Grade 4 thrombocytopenia and pleural effusion, Grade 3 pneumonia, anemia, and ascites. ** Group B: DLT.
This Phase 1b study is continuing to enroll expansion cohorts in r/r FL and MCL to further evaluate the combination of zandelisib 60 mg administered on days 1-7 starting Cycle 1 and zanubrutinib administered at 80 mg bid.
COASTAL Phase 3 Study Design
- Poster title: Coastal: A phase 3 study of the PI3Kδ inhibitor zandelisib with rituximab (R) versus immunochemotherapy in patients with relapsed indolent non-Hodgkin's lymphoma (iNHL)
- Available for on-demand viewing online: Beginning on June 4, 2021 at 6:00 a.m. PT here.
- Poster will also be available for download via the MEI Pharma website
The global, randomized, two-arm Phase 3 study will compare zandelisib plus rituximab to standard of care chemotherapy plus rituximab, is expected to enroll 534 patients, and the primary efficacy endpoint is progression-free survival. Zandelisib will be administered once daily for two 28-day cycles followed by an intermittent schedule of once daily dosing for one week of each subsequent 28-day cycle.
"As a potent, highly selective inhibitor of the PI3Kδ isoform, zandelisib has a differentiated therapeutic profile that makes it an ideal candidate to evaluate in different B-cell malignancies, both as a monotherapy and combined with other agents" said Professor Wojciech Jurczak, M.D., Ph.D., COASTAL principal investigator, Department of Clinical Oncology, Maria Sklodowska-Curie National Research Institute of Oncology in Kraków, Poland. "The initiation of the COASTAL study will be an important milestone as we evaluate zandelisib's potential as a best-in-class PI3Kδ therapy, optimized by a unique dosing schedule."
Clinical study of zandelisib as a monotherapy or in combination with other agents support its clinical development for B-cell malignancies. In clinical studies, zandelisib administered as a 2-month induction followed by intermittent schedule maintenance dosing achieved an 83
About Zandelisib
Zandelisib (formerly called ME-401), a selective PI3Kδ inhibitor, is an investigational cancer treatment being developed as an oral, once-daily, treatment for patients with B-cell malignancies. In March 2020 the U.S. FDA granted zandelisib Fast Track designation for treatment of adult patients with relapsed or refractory follicular lymphoma who have received at least 2 prior systemic therapies.
In April 2020, MEI and Kyowa Kirin Co., Ltd. (Kyowa Kirin) entered a global license, development, and commercialization agreement to further develop and commercialize zandelisib. MEI and Kyowa Kirin are co-developing and co-promoting zandelisib in the U.S., with MEI booking all revenue from the U.S. sales. Kyowa Kirin has exclusive commercialization rights outside of the U.S.
Ongoing clinical studies of zandelisib include TIDAL (Trials of PI3K DeltA in Non-Hodgkin's Lymphoma), a global Phase 2 study evaluating zandelisib as a monotherapy across two study arms: the first study arm for the treatment of adults with relapsed and refractory follicular lymphoma and the second study arm for marginal zone lymphomas, in both cases after failure of at least two prior systemic therapies including chemotherapy and an anti-CD20 antibody. The primary endpoint of the study is the objective response rate. Subject to the results and discussions with FDA, data from each study arm are intended to be submitted to FDA to support separate accelerated approval marketing applications under 21 CFR Part 314.500, Subpart H.
Also ongoing is a Phase 2 pivotal study in Japan in patients with indolent B-cell non-Hodgkin's lymphoma (iNHL) without small lymphocytic lymphoma (SLL), lymphoplasmacytic lymphoma (LPL), and Waldenström's macroglobulinemia (WM) conducted by Kyowa Kirin.
About PI3K Delta
Phosphatidylinositol 3-kinase delta ("PI3Kδ") is often overexpressed in cancer cells and plays a key role in the proliferation and survival of hematologic cancers. Zandelisib displays high selectivity for the PI3K delta isoform and has distinct pharmaceutical properties from other PI3K delta inhibitors.
About Follicular Lymphoma
Follicular lymphoma (FL) is the most common indolent lymphoma, comprising about 20
About MEI Pharma
MEI Pharma, Inc. (Nasdaq: MEIP) is a late-stage pharmaceutical company focused on developing potential new therapies for cancer. MEI Pharma's portfolio of drug candidates contains four clinical-stage assets, including zandelisib, currently in an ongoing Phase 2 clinical trial which may support accelerated approval applications with the U.S. Food and Drug Administration. Each of MEI Pharma's pipeline candidates leverages a different mechanism of action with the objective of developing therapeutic options that are: (1) differentiated, (2) address unmet medical needs and (3) deliver improved benefit to patients either as standalone treatments or in combination with other therapeutic options. For more information, please visit www.meipharma.com. Follow us on Twitter @MEI_Pharma and on LinkedIn.
About Kyowa Kirin
Kyowa Kirin strives to create and deliver novel medicines with life-changing value. As a Japan-based Global Specialty Pharmaceutical Company with a 70-year heritage, we apply cutting-edge science including an expertise in antibody research and engineering, to address the needs of patients and society across multiple therapeutic areas including Nephrology, Oncology, Immunology/Allergy and Neurology. Across our four regions – Japan, Asia Pacific, North America and EMEA/International – we focus on our purpose, to make people smile, and are united by our shared values of commitment to life, teamwork/Wa, innovation, and integrity. You can learn more about the business of Kyowa Kirin at: https://www.kyowakirin.com.
Forward-Looking Statements
Under U.S. law, a new drug cannot be marketed until it has been investigated in clinical studies and approved by the FDA as being safe and effective for the intended use. Statements included in this press release that are not historical in nature are "forward-looking statements" within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. You should be aware that our actual results could differ materially from those contained in the forward-looking statements, which are based on management's current expectations and are subject to a number of risks and uncertainties, including, but not limited to, our failure to successfully commercialize our product candidates; costs and delays in the development and or FDA approval, or the failure to obtain such approval, of our product candidates; uncertainties or differences in interpretation in clinical trial results; the impact of the COVID-19 pandemic on our industry and individual companies, including on our counterparties, the supply chain, the execution of our clinical development programs, our access to financing and the allocation of government resources; our inability to maintain or enter into, and the risks resulting from our dependence upon, collaboration or contractual arrangements necessary for the development, manufacture, commercialization, marketing, sales and distribution of any products; competitive factors; our inability to protect our patents or proprietary rights and obtain necessary rights to third party patents and intellectual property to operate our business; our inability to operate our business without infringing the patents and proprietary rights of others; general economic conditions; the failure of any products to gain market acceptance; our inability to obtain any additional required financing; technological changes; government regulation; changes in industry practice; and one-time events. We do not intend to update any of these factors or to publicly announce the results of any revisions to these forward-looking statements.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/mei-pharma-and-kyowa-kirin-announce-new-clinical-data-on-zandelisib-at-american-society-of-clinical-oncology-annual-meeting-2021-301295383.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/mei-pharma-and-kyowa-kirin-announce-new-clinical-data-on-zandelisib-at-american-society-of-clinical-oncology-annual-meeting-2021-301295383.html
SOURCE MEI Pharma, Inc.