Medtronic receives FDA approval for extravascular defibrillator to treat abnormal heart rhythms, sudden cardiac arrest
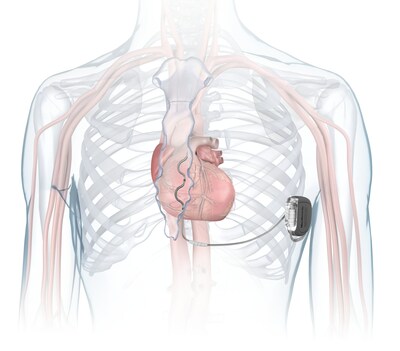
- The Aurora EV-ICD system provides the life-saving benefits of traditional ICDs with a lead placed outside of the heart and veins.
- The device's effectiveness in delivering defibrillation therapy at implant was 98.7%, with no major complications observed.
- At six months, 92.6% of patients were free from major complications such as hospitalization, system revision, or death.
- The Aurora EV-ICD system is nearly half the size and has 60% greater projected battery longevity compared to competitor's subcutaneous ICD.
- Patients can benefit from the only ICD placed outside the vascular space that provides ATP and back-up pacing.
- The system includes features such as anti-tachycardia pacing, pause prevention pacing, and 40 Joule defibrillation energy.
- The Aurora EV-ICD system is commercially available on a limited basis in the United States.
- Real-world performance and safety data on the Aurora system will be obtained through the Enlighten global post-approval registry.
Insights
Analyzing...
First-of-its-kind Aurora EV-ICD™ system offers single device, single procedure with lead placed outside of heart and veins
FDA approval of the Medtronic Aurora EV-ICD system includes the system's proprietary procedure implant tools, and was supported by global pivotal trial results showing the system's safety and effectiveness, which were published in The New England Journal of Medicine. In the coming weeks, the Aurora EV-ICD system will be commercially available on a limited basis in
"The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology," said Bradley P. Knight, M.D., medical director of Electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute and a co-author of the study. "Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology."
In the pivotal study, the device's effectiveness in delivering defibrillation therapy at implant was
ICDs are highly effective in providing life-saving therapy for patients at risk of SCA, an electrical problem with the heart due to a dangerously fast heart rate (ventricular tachycardia) or irregular rhythm (ventricular fibrillation). If not treated immediately, SCA is often fatal (termed sudden cardiac death). Traditional ICDs typically are implanted below the collarbone, with the lead(s) threaded through the veins and into the heart.
"This FDA approval paves the way for patients to have a better overall experience with ICD therapy," said Alan Cheng, M.D., chief medical officer of the Cardiac Rhythm Management business, which is part of the Cardiovascular Portfolio at Medtronic. "ICDs remain the gold standard for prevention of sudden cardiac death, and while the subcutaneous ICD avoids certain complications associated with transvenous defibrillators, it has limitations that may affect a patient's comfort and quality-of-life. With the Aurora EV-ICD system, patients can benefit from the only ICD placed outside the vascular space that provides ATP and back-up pacing, in a device that is nearly half the size and with
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional, transvenous ICDs. Unlike traditional ICDs, the Aurora EV-ICD system is implanted below the left armpit (in the left mid-axillary region) and the lead is placed under the breastbone (sternum) using a minimally invasive approach. The Epsila EV defibrillation lead is placed outside of the heart and veins, helping to avoid certain complications associated with transvenous leads, such as vascular injury and vessel occlusion (narrowing, blockage or compression of a vein).
The Aurora EV-ICD system includes features available in Medtronic transvenous ICDs, and offers additional advantages that are not available with the subcutaneous ICD including:
- Anti-tachycardia Pacing (ATP), to terminate ventricular arrhythmias (rapid and/or chaotic activity of the heart that can lead to SCA) using low-energy pacing pulses, potentially avoiding a defibrillation shock.
- Pause Prevention Pacing, to provide back-up pacing for brief, intermittent, heartbeat pauses.
- 40 Joule Defibrillation Energy, to deliver life-saving shocks in a device the size of transvenous ICDs (33 cc)
- Medtronic exclusive PhysioCurve™ design, to increase patient comfort and implant acceptance.
- 11.7-year projected longevity, to reduce device replacement procedures during a patient's lifetime.
Patients who receive the commercially available Aurora device also will benefit from the addition of Smart Sense, a proprietary algorithm designed to reduce the potential for inappropriate shocks.
Medtronic will obtain real-world performance and safety data on the Aurora system in the Enlighten global post-approval registry, a prospective, non-randomized, observational, multicenter study, expected to last five years and enroll approximately 1,000 patients. First implants in Enlighten and first commercial implants worldwide were recently conducted by Lucas V.A. Boersma, M.D., Ph.D., cardiologist at St. Antonius Hospital, Nieuwegein,
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, and who have not had a prior sternotomy and do not need chronic bradycardia (abnormally slow heartbeat) pacing.
About the EV ICD Pivotal Study
The EV ICD Pivotal study is a prospective, multicenter, single-arm, non-randomized, pre-market clinical study that assessed the safety and effectiveness of the Medtronic EV ICD system for patients at risk of sudden cardiac death. It enrolled 356 patients at 46 sites in 17 countries in
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
-end-
Contacts: | |
Tracy McNulty | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-763-526-2492 | +1-763-505-4626 |
______________________________________________
1Friedman P, Murgatroyd F, Boersma LVA, et al. Efficacy and Safety of an Extravascular Implantable Cardioverter-Defibrillator. N Engl J Med 2022; 387:1292-1302.
2Friedman P, Murgatroyd F, Boersma LVA, et al. Chronic Safety and Performance of the Extravascular ICD: Results from the Global EV ICD Pivotal Study. Heart Rhythm Society late breaking clinical trials, May 20, 2023.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-receives-fda-approval-for-extravascular-defibrillator-to-treat-abnormal-heart-rhythms-sudden-cardiac-arrest-301963889.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-receives-fda-approval-for-extravascular-defibrillator-to-treat-abnormal-heart-rhythms-sudden-cardiac-arrest-301963889.html
SOURCE Medtronic plc