Medtronic Receives Approval for U.S. Labeling of the Intellis™ Platform Showing Superior Back Pain Relief When Using DTM™ Spinal Cord Stimulation
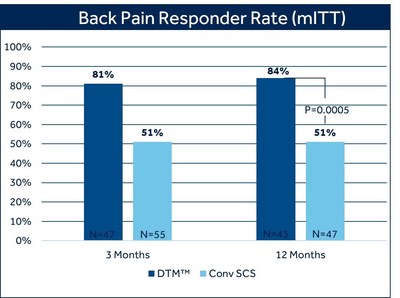
Medtronic plc (NYSE: MDT) announced the FDA's approval of revised commercial labeling for its Intellis™ Platform, showcasing the efficacy of Differential Target Multiplexed (DTM™) programming in treating chronic back pain. A randomized control trial revealed that 80% of patients using DTM SCS experienced over 50% back pain relief after three months, compared to 51% with conventional SCS. At twelve months, 84% reported significant pain relief with DTM SCS. This update strengthens Medtronic's credibility in pain management solutions.
- FDA approval of updated labeling for Intellis Platform reflects strong clinical trial results.
- DTM SCS programming showed superior pain relief outcomes with 80% of patients reporting over 50% relief at 3 months.
- Long-term data supports efficacy with 84% of patients experiencing significant pain relief after 12 months.
- None.
Insights
Analyzing...
DUBLIN, March 17, 2021 /PRNewswire/ -- Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced the U.S. Food and Drug Administration (FDA) has approved revised commercial labeling for the Intellis™ Platform with Differential Target Multiplexed (DTM™) programming for the treatment of chronic, intractable back and leg pain. The new labeling will include study outcomes from a multicenter randomized control trial reflecting superior back pain relief with DTM SCS when compared to conventional SCS.
The trial demonstrated with clinical and statistical significance that DTM SCS programming is superior to conventional SCS programming when used to treat intractable chronic back pain. At 3 months,
This labeling update closely follows the most recent presentation of 12-month clinical trial outcomes during a late-breaking clinical trial session at NANS 2021.2 Trial results showed statistically significant and superior back pain relief with DTM SCS compared to conventional SCS at 12 months:
"The body of clinical evidence proving the efficacy of DTM SCS in treating patients with chronic back pain continues to grow," said Charlie Covert, vice president and general manager, Pain Therapies within the Neuromodulation business, which is part of the Neuroscience Portfolio at Medtronic. "The updated labeling further strengthens the credibility of the outcomes from this therapy, and parallels the profound benefits our clinician partners are seeing with their own patients."
DTM therapy, which is clinically proven and only available on the Medtronic Intellis platform, is a unique and proprietary programming option available to treat patients with chronic pain that is based on years of preclinical research.3,4,5
Clinical trial data can be found www.medtronic.com/DTM. A manuscript has been submitted for consideration for peer-reviewed publication.
About the Intellis™ Platform
The Intellis platform offers the world's smallest implantable neurostimulator. It is powered by proprietary Overdrive™ battery technology and was designed to overcome limitations with other SCS systems, optimized for a wide range of energy demands and provides effective long-term pain relief for patients. The neurostimulator also features SureScan™ MRI technology, allowing access to MRI anywhere in the body under certain conditions, and AdaptiveStim™ technology, which automatically adjusts stimulation based on the patient's needs and preferences in different body positions to ensure the patient receives the right dose of stimulation at the right location.
About Medtronic Pain Therapies
Medtronic has more than a 40-year history of developing innovative medical devices that have been shown to alleviate pain in different disease states and has a broad portfolio of device-delivered therapies that are alternatives or adjuncts to oral opioids.4 Medtronic strives to be at the forefront of medical device innovation and to develop high-quality pain therapies that reduce pain and improve quality of life. While Medtronic pain therapies do not treat opioid addiction, we are committed to leveraging our capabilities and product portfolio in partnership with stakeholders — patients, providers, payers, regulators, elected officials, patient advocacy groups and employers — to address the unmet needs of pain patients and to support efforts to prevent opioid misuse due to chronic intractable pain.
About Medtronic
Medtronic plc (www.medtronic.com), headquartered in Dublin, Ireland, is among the world's largest medical technology, services and solutions companies – alleviating pain, restoring health and extending life for millions of people around the world. Medtronic employs more than 90,000 people worldwide, serving physicians, hospitals and patients in more than 150 countries. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
References
- Fishman M, Cordner H, Justiz R, et al. Randomized Controlled Clinical Trial to Study the Effects of SGX-SCS in Treating Intractable Chronic Low Back Pain: 3 Month Results. North American Neuromodulation Society (NANS); 2020; Las Vegas, Nevada.
- Fishman M, Cordner H, Justiz R, et al. Randomized Controlled Clinical Trial to Study the Effects of Differential Target Multiplexed™ SCS (DTMTM SCS) in Treating Intractable Chronic Low Back Pain: Long-term Follow-Up Results. Presented at: North American Neuromodulation Society 24th Annual Meeting. Jan 15-16, 2021. Virtual.
- Vallejo R, Kelley CA, Gupta A, Smith WJ, Vallejo A, Cedeño DL. Modulation of neuroglial interactions using differential target multiplexed spinal cord stimulation in an animal model of neuropathic pain. Mol Pain. 2020 Jan-Dec;16:1744806920918057.http://dx.doi.org/10.1177/1744806920918057
- Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010 May-Jun;10(3):167-84.
- Cedeno DL, Smith WJ, Kelley CA, Vallejo R. Spinal cord stimulation using differential target multiplexed programming modulates neural cell-specific transcriptomes in an animal model of neuropathic pain. Mol Pain. 2020;16:1744806920964360. http://dx.doi.org/10.1177/1744806920964360
Contacts: | |
Jeff Trauring | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-763-505-0159 | +1-763-505-4626 |
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/medtronic-receives-approval-for-us-labeling-of-the-intellis-platform-showing-superior-back-pain-relief-when-using-dtm-spinal-cord-stimulation-301249014.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/medtronic-receives-approval-for-us-labeling-of-the-intellis-platform-showing-superior-back-pain-relief-when-using-dtm-spinal-cord-stimulation-301249014.html
SOURCE Medtronic plc