Medtronic Launches the First and Only Pediatric and Neonatal Acute Dialysis Machine in the U.S.
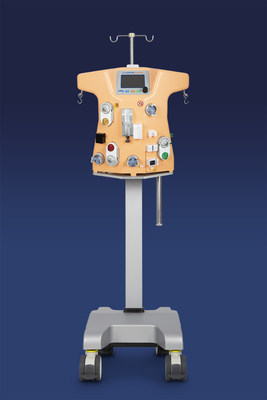
Medtronic plc (NYSE:MDT) has launched the Carpediem™ Cardio-Renal Pediatric Dialysis Emergency Machine in the U.S. following FDA marketing authorization. This innovative system, designed specifically for patients weighing 2.5 to 10 kilograms, addresses the critical needs of young patients with acute kidney injury or fluid overload, providing safer and more effective hemodialysis. The first units are now in use at Cincinnati Children's Hospital Medical Center, enhancing the treatment options for neonates, who face a high mortality rate from kidney injuries.
- Launch of the Carpediem™ system enhances treatment for pediatric patients with acute kidney injury.
- First system in the U.S. designed specifically for patients between 2.5 and 10 kg.
- Successful implementation at Cincinnati Children's Hospital, indicating immediate clinical adoption.
- None.
Insights
Analyzing...
DUBLIN, Dec. 8, 2020 /PRNewswire/ -- Medtronic plc (NYSE:MDT) today announced the U.S. commercial launch of the Carpediem™ Cardio-Renal Pediatric Dialysis Emergency Machine. Following the U.S. Food and Drug Administration's (FDA) marketing authorization, granted earlier this year, the first Carpediem™ systems in the United States were successfully installed and are in use at Cincinnati Children's Hospital Medical Center. The first of its kind Carpediem™ system is indicated for use in acute kidney injury or fluid overloaded patients requiring hemodialysis or hemofiltration therapy. It is intended to provide continuous renal replacement therapy (CRRT) to patients weighing between 2.5 and 10 kilograms.
Critically ill patients are at a high risk for fluid overload and acute kidney injury, conditions in which the kidneys do not function properly in their vital role of filtering waste products and excess fluid from the blood to produce urine. Fluid overload is common in critically ill neonates and children, particularly after procedures such as cardiac surgery. The mortality rate for neonates with acute kidney injury has been reported to be as high as 60 percent.[1]
"CRRT procedures performed for critically ill infants using previously available technology are not optimal largely because dialysis machines available in the U.S. are not designed to treat these small, fragile patients, and can potentially expose them to many risks," said Stuart L. Goldstein, M.D., professor of pediatrics and director, Center for Acute Care Nephrology at Cincinnati Children's Hospital Medical Center, who has been instrumental in raising awareness of the critical need for safe pediatric-specific dialysis. "This new system is designed specifically for these patients which enables increased precision of neonatal CRRT treatment and, potentially, reduces these risks. We are grateful to be the first site in the U.S. with this technology to help the children in our care."
CRRT is the most common treatment for critically ill patients whose kidneys are not functioning properly. In this form of renal replacement therapy, the patient's blood is pumped through a hemofilter to gently remove waste and excess fluid while minimizing the risk for hypotension (low blood pressure) and cardiac stability.
Pediatric patients requiring CRRT have historically been treated with systems designed and indicated for adults and not approved for pediatric use, which can create potential clinical complications for neonatal patients. The Carpediem™ system is intended to address many of the challenges associated with current machines because it is the first CRRT system designed specifically for patients weighing between 2.5 and 10 kilograms. This system was championed by Professor Claudio Ronco, Director, Department of Nephrology and International Renal Research Institute of the San Bortolo Hospital, Vicenza, Italy (IRRIV).
"At Medtronic, we strive to provide a portfolio of renal care solutions that improve outcomes, access to care, and quality of life for patients affected by severe renal injury or disease globally — no matter their size or age," said Ven Manda, president, Renal Care Solutions, which is reported as part of the Medtronic Minimally Invasive Therapies Group. "For the first time, some of the tiniest and most vulnerable patients can be treated with technology designed specifically for them. We cannot make the world a healthier place alone. That is why collaboration with clinical experts, such as Prof. Ronco and Dr. Goldstein, is critical to bringing new treatment options to underserved populations."
About Medtronic
Medtronic plc (www.medtronic.com), headquartered in Dublin, Ireland, is among the world's largest medical technology, services and solutions companies - alleviating pain, restoring health and extending life for millions of people around the world. Medtronic employs more than 90,000 people worldwide, serving physicians, hospitals and patients in approximately 160 countries. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
[1] Zapitelli M, Manasivayam A, Askenazi DJ, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res. 2017;82(4):569-573. doi: 10.1038/pr.2017.136. Epub 2017 Jul 11.
Contacts: | |
Kira Jastive Public Relations +1-508-452-4238 | Ryan Weispfenning Investor Relations +1-763-505-4626 |
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/medtronic-launches-the-first-and-only-pediatric-and-neonatal-acute-dialysis-machine-in-the-us-301188191.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/medtronic-launches-the-first-and-only-pediatric-and-neonatal-acute-dialysis-machine-in-the-us-301188191.html
SOURCE Medtronic plc