New Study Evaluates the Utility of Masimo ORi™ in Reducing Hyperoxemia During Laparoscopic Gastrectomy
Masimo (NASDAQ: MASI) announced findings from a study published in Medicine, showing that using the Oxygen Reserve Index (ORi™) alongside standard SpO2 monitoring effectively reduced intraoperative hyperoxemia during laparoscopic gastrectomy. Conducted by Dr. Jin Hee Ahn and team, the study demonstrated significant differences in arterial oxygen levels; the SpO2 group had a higher PaO2 compared to the ORi-SpO2 group one hour post-surgery (250.31 mmHg vs. 170.07 mmHg, p < .001). The severe hyperoxemia incidence was lower in the ORi-SpO2 group at 16.7% vs. 84.4% for SpO2.
- Study shows ORi combined with SpO2 monitoring significantly reduces intraoperative hyperoxemia.
- Demonstrated lower rates of severe hyperoxemia in patients guided by ORi (16.7% vs. 84.4%).
- Highlights the clinical relevance of ORi in surgical settings.
- ORi has not received FDA clearance and is not available in the U.S.
Insights
Analyzing...
Researchers Found That Using ORi with SpO2 to Help Guide Supplemental Oxygen Delivery Reduced Intraoperative Hyperoxemia
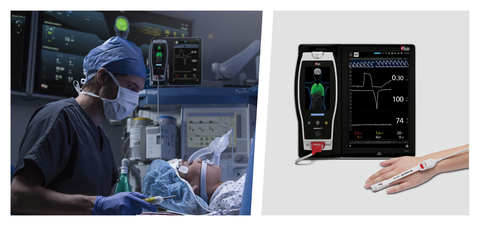
Masimo Root® with ORi™ (Photo: Business Wire)
ORi, available outside the
Noting that the use of supplemental oxygen during general surgery increases the risk of hyperoxemia, the researchers sought to evaluate whether noninvasive, continuous ORi might improve clinicians’ ability to detect hyperoxemia, since SpO2 monitoring alone cannot monitor beyond
In the ORi-SpO2 group, FiO2 was adjusted to maintain ORi > 0 and < 0.3, which was evaluated every 2 to 3 minutes throughout surgery. In the SpO2 group, FiO2 was adjusted to maintain SpO2 ≥
The researchers found that one hour after surgical incision, PaO2 was higher in the SpO2 group (250.31 ± 57.39 mmHg) than in the ORi-SpO2 group (170.07 ± 49.39 mmHg) (p < .001), and remained consistently higher in the SpO2 group than in the ORi-SpO2 group over time (p = .045). The rate of severe hyperoxemia was higher in the SpO2 group (
The researchers concluded, “[I]ntraoperative hyperoxemia was reduced when FiO2 was adjusted based on the combination of SpO2 and ORi compared with SpO2 alone in patients undergoing laparoscopic gastrectomy.”
ORi has not yet received FDA clearance and is not available in
@Masimo | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23
ORi, RPVi, and Radius VSM have not received FDA 510(k) clearance and are not available for sale in
References
-
Ahn JH, Shim J-G, Park J, Lee SH, Ryu K-H, Cho E-A. Oxygen reserve index guided fraction of inspired oxygen titration to reduce hyperoxemia during laparoscopic gastrectomy: A randomized controlled trial. Medicine.
October 2022 . DOI: 10.1097/MD.0000000000031592. - Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
-
McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation.
The Joint Commission Journal on Quality and Patient Safety . 2016 Jul;42(7):293-302. - McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™, SET®, rainbow®, and Radical-7®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including
View source version on businesswire.com: https://www.businesswire.com/news/home/20230220005333/en/
Media Contact:
Masimo
949-396-3376
elamb@masimo.com
Source: Masimo







