Masimo Announces Limited Market Release of Sepsis Index
Masimo (NASDAQ: MASI) has announced the limited market release of Sepsis Index (Si™), an early warning solution for identifying potential sepsis in patients monitored with Masimo Patient SafetyNet™. This system integrates real-time physiological data to provide a sepsis risk score, allowing clinicians to detect and respond to patient deterioration more effectively. Sepsis affects approximately 1.7 million adults annually in the U.S. with significant mortality rates. The Sepsis Index aims to improve patient outcomes through timely interventions, although it is not FDA cleared.
- Launch of Sepsis Index (Si™) for early sepsis detection.
- Utilizes real-time data from Masimo Patient SafetyNet™, enhancing patient monitoring.
- Addresses a critical healthcare issue, with sepsis causing over 350,000 deaths annually in the U.S.
- Potential for improved hospital resource allocation and patient outcomes.
- Sepsis Index (Si™) is not FDA cleared, limiting its initial use.
Breakthrough Early Warning Solution, for Integration with Masimo Patient SafetyNet™, Is Designed to Help Clinicians Catch Deterioration in Patient Condition
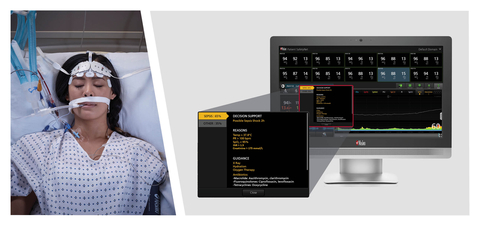
Masimo Patient SafetyNet™ with Sepsis Index (Si™) (Photo: Business Wire)
Sepsis Index, using the aggregated data from Patient SafetyNet, is designed to provide early warning of possible sepsis or other causes of deterioration in a patient’s condition, and to help track patient status following sepsis diagnosis and monitor the effectiveness of treatment and interventions. In addition, Si is designed to display on-screen decision support and recommended actions that each institution can update according to its sepsis policy. The Si algorithm is customizable and draws upon Masimo’s expertise in developing a variety of advanced early warning risk-scoring solutions, such as Halo and Halo ION®, since 2009.
Sepsis, a life-threatening condition in which organs malfunction because of widespread infection, is not only common among hospital patients and is a common cause of death.1 The
Sepsis Index (Si) is not FDA cleared.
@Masimo | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.5 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,6 improve CCHD screening in newborns,7 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.8-11 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,12 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23
ORi, RPVi, and Radius VSM have not received FDA 510(k) clearance and are not available for sale in
References
- Singer M et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016.
-
https://www.cdc.gov/patientsafety/features/get-ahead-of-sepsis.html#:~:text=About%
201.7% 20million%20adults%20in,had%20sepsis%20during%20that%20hospitalization . - https://www.sepsis.org/news/new-u-s-government-report-reveals-annual-cost-of-hospital-treatment-of-sepsis-has-grown-by-3-4-billion/
-
Kim HI,
Park S . Sepsis: Early Recognition and Optimized Treatment. Tuberc Respir Dis (Seoul ). 2019 Jan;82(1):6-14. doi: 10.4046/trd.2018.0041. Epub 2018 Sep 28. PMID: 30302954; PMCID: PMC6304323. - Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
-
McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation.
The Joint Commission Journal on Quality and Patient Safety . 2016 Jul;42(7):293-302. - McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Si™ and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including
View source version on businesswire.com: https://www.businesswire.com/news/home/20221211005045/en/
Masimo
949-396-3376
elamb@masimo.com
Source: Masimo
FAQ
What is Masimo's Sepsis Index (Si™)?
How does Sepsis Index (Si™) work?
What are the statistics on sepsis in the U.S.?
Is Sepsis Index (Si™) FDA cleared?







