Lantern Pharma Presents Positive Data on the Effectiveness of LP-284 in Hematologic Cancers at the 63rd American Society of Hematology (ASH) Annual Meeting
On December 14, 2021, Lantern Pharma (NASDAQ: LTRN) announced positive data on LP-284's efficacy against hematologic cancers presented at the ASH Annual Meeting. LP-284, a synthetic acylfulvene compound, showcased broad anti-tumor activity against lymphoma, multiple myeloma, and leukemia in vitro. The findings suggest LP-284 could serve as a targeted therapy option for cancers with DNA repair deficiencies. Lantern plans to leverage its RADR® A.I. platform to advance LP-284 development and address the urgent need for new treatment options in blood cancers.
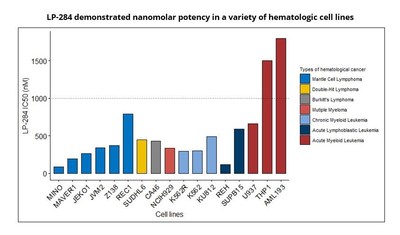
- LP-284 demonstrated broad in vitro anti-tumor activity in hematological cancers.
- Data supports LP-284's potential as a targeted therapy for cancers with compromised DNA repair.
- Lantern aims to expedite LP-284's development leveraging its RADR® A.I. platform.
- LP-284 has not yet received FDA marketing approval.
- No guarantee of successful clinical testing for LP-284.
Insights
Analyzing...
DALLAS, Dec. 14, 2021 /PRNewswire/ -- Lantern Pharma (NASDAQ: LTRN), a clinical stage biopharmaceutical company using its proprietary RADR® artificial intelligence ("A.I.") platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced that Lantern Pharma presented positive data on the effectiveness of LP-284 in hematologic cancers at the 63rd American Society of Hematology (ASH) Annual Meeting, which was held in-person and virtually from December 11 – 14, 2021. This poster presentation can be viewed on Lantern Pharma's website at: https://www.lanternpharma.com/about/publications.
LP-284 is a fully synthetic molecule belonging to the new generation of acylfulvenes, a family of naturally derived anti-cancer drug candidates. LP-284 is the stereoisomer (enantiomer) of LP-184 and has the potential for development as monotherapy and also as a synergistic agent in combination with other drugs. LP-284 is currently being evaluated for activity in a wide spectrum of hematological cancers. Earlier this year, Lantern filed multi-national patent applications directed to both the composition and manufacture of LP-284.
The study demonstrated LP-284's broad in vitro anti-tumor activity in lymphoma, multiple myeloma, and leukemia cells. Notably, the enantiomer pair, LP-184 and LP-284, exhibit distinct patterns of anti-tumor activities. As a result, the novel enantiomer LP-284 may provide a targeted therapy option for hematologic cancers with compromised DNA repair, supporting further targeted development plans for LP-284.
"Approximately every 3 minutes, one person in the U.S. is diagnosed with leukemia, lymphoma or myeloma and approximately every 9 minutes someone in the U.S. dies from a blood cancer1," commented Panna Sharma, CEO and President of Lantern Pharma Inc. "There is an urgent need to develop new, targeted therapies; however, the current industry approach is time consuming and expensive. This latest data provides further validation of Lantern's RADR® platform, which leverages A.I. and machine learning with the aim of dramatically shortening the timeline and reducing costs associated with drug discovery and development. Specifically, these findings support our hypothesis that LP-284 has the potential to become a targeted therapy option for hematologic cancers with compromised DNA repair. We plan to apply the insights obtained from this work to advance the development of LP-284 in rare blood cancer indications. This data is very encouraging, and we look forward to advancing LP-284 towards the clinic, while leveraging our RADR® platform to develop new cutting-edge treatments for rare blood cancers and other indications."
Lantern's proprietary RADR® A.I. platform leverages over 10 billion data points, machine learning, genomics, and computational biology to accelerate the discovery of potential mechanisms of action, and biomarker signatures that correlate to drug response in rare blood cancers.
About Lantern Pharma
Lantern Pharma (NASDAQ: LTRN) is a clinical-stage oncology-focused biopharmaceutical company leveraging its proprietary RADR® A.I. platform and machine learning to discover biomarker signatures that identify patients most likely to respond to its pipeline of genomically-targeted therapeutics. Lantern is currently developing four drug candidates and an ADC program across eight disclosed tumor targets, including two phase 2 programs. By targeting drugs to patients whose genomic profile identifies them as having the highest probability of benefiting from the drug, Lantern's approach represents the potential to deliver best-in-class outcomes. More information is available at: www.lanternpharma.com and Twitter @lanternpharma.
About RADR®
RADR® or Response Algorithm for Drug Positioning & Rescue, is Lantern's proprietary integrated A.I. platform for large-scale biomarker and drug-tumor interaction data analytics that leverages machine-learning. RADR® is used to provide mechanistic insights about drug-tumor interactions, predict the potential response of cancer types and subtypes to existing drugs and drug candidates, and uncover patient groups that may respond to potential therapies being developed by Lantern and its collaborators.
Forward-looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, among other things, statements relating to: future events or our future financial performance; the potential advantages of our RADR® platform in identifying drug candidates and patient populations that are likely to respond to a drug candidate; our strategic plans to advance the development of our drug candidates and antibody drug conjugate (ADC) development program; estimates regarding the development timing for our drug candidates and ADC development program; our research and development efforts of our internal drug discovery programs and the utilization of our RADR® platform to streamline the drug development process; our intention to leverage artificial intelligence, machine learning and genomic data to streamline and transform the pace, risk and cost of oncology drug discovery and development and to identify patient populations that would likely respond to a drug candidate; estimates regarding potential markets and potential market sizes; sales estimates for our drug candidates and our plans to discover and develop drug candidates and to maximize their commercial potential by advancing such drug candidates ourselves or in collaboration with others. Any statements that are not statements of historical fact (including, without limitation, statements that use words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "objective," "aim," "upcoming," "should," "will," "would," or the negative of these words or other similar expressions) should be considered forward-looking statements. There are a number of important factors that could cause our actual results to differ materially from those indicated by the forward-looking statements, such as (i) the impact of the COVID-19 pandemic, (ii) the risk that our research and the research of our collaborators relating to LP-284 may not be successful, (iii) the risk that none of our product candidates has received FDA marketing approval, and we may not be able to successfully initiate, conduct, or conclude clinical testing for or obtain marketing approval for our product candidates, (iv) the risk that no drug product based on our proprietary RADR A.I. platform has received FDA marketing approval or otherwise been incorporated into a commercial product, and (v) those other factors set forth in the Risk Factors section in our Annual Report on Form 10-K for the year ended December 31, 2020, filed with the Securities and Exchange Commission on March 10, 2021. You may access our Annual Report on Form 10-K for the year ended December 31, 2020 under the investor SEC filings tab of our website at www.lanternpharma.com or on the SEC's website at www.sec.gov. Given these risks and uncertainties, we can give no assurances that our forward-looking statements will prove to be accurate, or that any other results or events projected or contemplated by our forward-looking statements will in fact occur, and we caution investors not to place undue reliance on these statements. All forward-looking statements in this press release represent our judgment as of the date hereof, and, except as otherwise required by law, we disclaim any obligation to update any forward-looking statements to conform the statement to actual results or changes in our expectations.
CONTACT:
Investor Relations
David Waldman/Natalya Rudman, Crescendo Communications, LLC
IR@lanternpharma.com
212-671-1021
1 https://www.lls.org/facts-and-statistics/facts-and-statistics-overview
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/lantern-pharma-presents-positive-data-on-the-effectiveness-of-lp-284-in-hematologic-cancers-at-the-63rd-american-society-of-hematology-ash-annual-meeting-301444227.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/lantern-pharma-presents-positive-data-on-the-effectiveness-of-lp-284-in-hematologic-cancers-at-the-63rd-american-society-of-hematology-ash-annual-meeting-301444227.html
SOURCE Lantern Pharma