Modified Titration of Donanemab Demonstrated Reduction of ARIA-E in Early Symptomatic Alzheimer's Disease Patients in Phase 3b study
Rhea-AI Summary
Eli Lilly announced positive results from the TRAILBLAZER-ALZ 6 Phase 3b study for Kisunla (donanemab) in early symptomatic Alzheimer's disease. A modified titration dosing regimen, which shifted one vial from the first infusion to the third infusion, reduced ARIA-E incidence to 14% compared to 24% with standard dosing. The reduction was most significant in APOE4 homozygotes, dropping from 57% to 19%. The modified regimen maintained comparable amyloid plaque reduction (67% vs 69%) and P-tau217 levels. Lilly plans to submit this data to regulators for a potential label update.
Positive
- Modified dosing reduced ARIA-E incidence by 41% (14% vs 24%)
- 67% reduction in ARIA-E risk for APOE4 homozygotes (19% vs 57%)
- Maintained efficacy with 67% amyloid plaque reduction from baseline
- Equivalent cumulative drug exposure between modified and standard dosing
Negative
- One death reported in modified titration arm due to cerebral hemorrhage after tPA treatment
- Safety concerns remain regarding interaction with thrombolytic agents
News Market Reaction
On the day this news was published, LLY gained 0.89%, reflecting a mild positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
A change in the initiation of donanemab dosing, shifting one vial of donanemab from the first infusion to the third infusion, lowered ARIA-E to
Reduction of amyloid plaque and P-tau217 on this modified titration was comparable to patients receiving the standard dosing regimen
Lilly intends to submit this data to global regulators for a potential label update for Kisunla (donanemab-azbt)
TRAILBLAZER-ALZ 6 is a multicenter, randomized, double-blind, Phase 3b study to investigate the impact of different dosing regimens of donanemab on the rates of ARIA-E and amyloid clearance in adults with early symptomatic AD, which includes mild cognitive impairment (MCI) and the mild dementia stage of disease.
"Lilly is committed to continuing research to optimize therapy for patients with Alzheimer's disease. We are confident in the benefits of Kisunla's currently approved dosing regimen and are excited that these results reveal a path to potentially improving on Kisunla's profile by reducing the risk of ARIA-E," said Mark Mintun, M.D., group vice president Neuroscience Research & Development, Eli Lilly and Company, and president of Avid Radiopharmaceuticals. "The modified titration could offer continued convenience of once-monthly dosing and limited duration treatment while also reducing ARIA-E and maintaining similar amyloid plaque removal."
The study included four treatment arms. One arm was the once-monthly standard dosing regimen used in the Phase 3 TRAILBLAZER-ALZ 2 trial. The other three arms used alternative dosing regimens with the same total drug administered. The results at 24 weeks showed the once-monthly modified titration described below met the predefined study success criteria and demonstrated the greatest reduction of ARIA-E compared to the other dosing arms.1
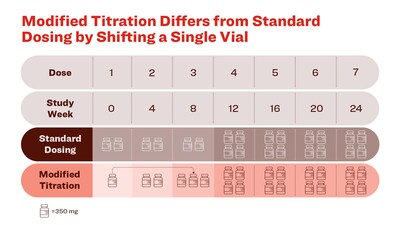
- Patients on the standard dosing regimen received two vials (700 mg) of donanemab for the first three infusions, then four vials (1400 mg) thereafter.
- Patients on the modified titration received 1 vial (350 mg) for the first infusion, two vials (700 mg) for the second infusion, three vials (1,050 mg) for the third infusion, and four vials (1400 mg) per infusion thereafter.
- The only difference between the modified titration and the standard dosing regimen is the shift of one vial from the first infusion to the third infusion.
- Pharmacokinetic (PK) analysis demonstrated equivalent cumulative exposure between modified titration and standard dosing regimen.
The primary endpoint of the study was the proportion of participants with any occurrence of ARIA-E by week 24, and the results showed the incidence of ARIA-E was
Patients on the modified titration of donanemab saw reduction of amyloid plaque and P-tau217 comparable to patients receiving the standard dosing regimen. As observed using amyloid PET at 24 weeks, amyloid plaque levels in patients on the modified titration of donanemab in TRAILBLAZER-ALZ 6 were reduced on average
The TRAILBLAZER-ALZ 6 study is ongoing with data investigating the potential reduction of ARIA-E in adults with early symptomatic AD at week 52 expected early next year. Aside from the improvement in ARIA-E rates, safety profiles were comparable across arms, and no new safety signals were identified. The frequency of infusion-related reactions in the modified titration arm was similar to the standard dosing arm. One participant in the modified titration arm with an ongoing ARIA-E presented stroke-like symptoms, and after receiving tissue-type plasminogen activator treatment (tPA), subsequently died due to cerebral intraparenchymal hemorrhage. As noted in the FDA labeling for this class of medicines, caution should be exercised when considering the administration of agents such as thrombolytics (e.g. tPA) to patients already being treated with amyloid targeting therapies.
Lilly is discussing the results of this study with global regulators, with the intent to submit for a potential label update for Kisunla.
About TRAILBLAZER-ALZ 6 Study and the TRAILBLAZER-ALZ program
TRAILBLAZER-ALZ 6 (NCT05738486) is a Phase 3b, multicenter, randomized, double-blind study to investigate different dosing regimens and their effect on ARIA-E in adults with early symptomatic Alzheimer's disease. The trial enrolled 843 participants ages 60-85 selected based on cognitive assessments in conjunction with amyloid plaque imaging by PET scan.
Lilly continues to study donanemab in multiple clinical trials, including TRAILBLAZER-ALZ 3, focused on reducing risk of progression to symptomatic AD in participants with preclinical AD; and TRAILBLAZER-ALZ 5, a registration trial for early symptomatic AD currently enrolling in China,
About Kisunla™ (donanemab)
Kisunla™ (donanemab-azbt) (pronounced kih-SUHN-lah) is an amyloid-targeting treatment for people with mild cognitive impairment (MCI) as well as people with mild dementia stage of early symptomatic Alzheimer's disease, with confirmed amyloid pathology. Kisunla can cause serious side effects, including amyloid-related imaging abnormalities, or ARIA, and infusion-related reactions. Kisunla is a prescription medicine administered intravenously every four weeks, 700 mg for the first three doses and 1400 mg thereafter.
INDICATION AND SAFETY SUMMARY WITH WARNINGS
Kisunla™ (donanemab-azbt), pronounced kih-SUHN-lah, is used to treat adults with early symptomatic Alzheimer's disease (AD), which includes mild cognitive impairment (MCI) or mild dementia stage of disease.
Warnings - Kisunla can cause Amyloid-Related Imaging Abnormalities or "ARIA." This is a common side effect that does not usually cause any symptoms, but serious symptoms can occur. ARIA can be fatal. ARIA is most commonly seen as temporary swelling in an area or areas of the brain that usually goes away over time. Some people may also have spots of bleeding on the surface of or in the brain and infrequently, larger areas of bleeding in the brain can occur. Although most people do not have symptoms, some people have headaches, dizziness, nausea, difficulty walking, confusion, vision changes and seizures.
Some people have a genetic risk factor (homozygous apolipoprotein E ε4 gene carriers) that may cause an increased risk for ARIA. Talk to your healthcare provider about testing to see if you have this risk factor.
You may be at higher risk of developing bleeding in the brain if you take medicines to reduce blood clots from forming (antithrombotic medicines) while receiving Kisunla. Talk to your healthcare provider to see if you are on any medicines that increase this risk.
Your healthcare provider will do magnetic resonance imaging (MRI) brain scans before and during your treatment with Kisunla to check you for ARIA. You should carry information that you are receiving Kisunla, which can cause ARIA, and that ARIA symptoms can look like stroke symptoms.
Call your healthcare provider or go to the nearest hospital emergency room right away if you have any of the symptoms listed above.
There are registries that collect information on treatments for Alzheimer's disease. Your healthcare provider can help you become enrolled in these registries.
Warnings - Kisunla can cause serious allergic and infusion-related reactions. Do not receive Kisunla if you have serious allergic reactions to donanemab-azbt or any of the ingredients in Kisunla. Symptoms may include swelling of the face, lips, mouth, or eyelids, problems breathing, hives, chills, irritation of skin, nausea, vomiting, sweating, headache, or chest pain. You will be monitored for at least 30 minutes after you receive Kisunla for any reaction. Tell your healthcare provider right away if you have these symptoms or any reaction during or after a Kisunla infusion.
Other common side effects
- Headache
Tell your healthcare provider right away if you have any side effects. These are not all of the possible side effects of Kisunla. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before you receive Kisunla, tell your healthcare provider:
- About all medicines you take, including prescription and over-the-counter medicines, as well as vitamins and herbal supplements. Especially tell your healthcare provider if you have medicines to reduce blood clots from forming (antithrombotic medicines, including aspirin).
- About all of your medical conditions including if you are pregnant, breastfeeding, or plan to become pregnant or breastfeed. Kisunla has not been studied in people who were pregnant or breastfeeding. It is not known if Kisunla could harm your unborn or breastfeeding baby.
How to receive Kisunla
Kisunla is a prescription medicine given through an intravenous (IV) infusion using a needle inserted into a vein in your arm. Kisunla is given once every 4 weeks. Each infusion will last about 30 minutes.
Learn more
For more information about Kisunla, call 1-800-LillyRx (1-800-545-5979) or go to kisunla.lilly.com.
This summary provides basic information about Kisunla. It does not include all information known about this medicine. Read the information given to you about Kisunla. This information does not take the place of talking with your healthcare provider. Be sure to talk to your healthcare provider about Kisunla. Your healthcare provider is the best person to help you decide if Kisunla is right for you.
Please see full Prescribing Information including boxed warning for ARIA and Medication Guide for Kisunla.
About Lilly
Lilly is a medicine company turning science into healing to make life better for people around the world. We've been pioneering life-changing discoveries for nearly 150 years, and today our medicines help tens of millions of people across the globe. Harnessing the power of biotechnology, chemistry and genetic medicine, our scientists are urgently advancing new discoveries to solve some of the world's most significant health challenges: redefining diabetes care; treating obesity and curtailing its most devastating long-term effects; advancing the fight against Alzheimer's disease; providing solutions to some of the most debilitating immune system disorders; and transforming the most difficult-to-treat cancers into manageable diseases. With each step toward a healthier world, we're motivated by one thing: making life better for millions more people. That includes delivering innovative clinical trials that reflect the diversity of our world and working to ensure our medicines are accessible and affordable. To learn more, visit Lilly.com and Lilly.com/news, or follow us on Facebook, Instagram and LinkedIn. P-LLY
© Lilly
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements (as that term is defined in the Private Securities Litigation Reform Act of 1995) about Kisunla (donanemab-azbt) as a treatment for people with early symptomatic Alzheimer's disease, Kisunla dosing regimens and the prevalence of ARIA-E, and future readouts, presentations, and other milestones relating to Kisunla and reflects Lilly's current beliefs and expectations. However, as with any pharmaceutical product, there are substantial risks and uncertainties in the process of drug research, development, and commercialization. Among other things, there is no guarantee that planned or ongoing studies will be completed as planned, that future study results will be consistent with study findings to date, that Kisunla will receive additional regulatory approvals or that Kisunla will be commercially successful. For further discussion of these and other risks and uncertainties, see Lilly's Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission. Except as required by law, Lilly undertakes no duty to update forward-looking statements to reflect events after the date of this release.
References
- Eli Lilly. A Study of Different Donanemab (LY3002813) Dosing Regimens in Adults With Early Alzheimer's Disease (TRAILBLAZER-ALZ 6). ClinicalTrials.gov identifier: NCT05738486. Updated July 24, 2024. Accessed September 30, 2024. https://clinicaltrials.gov/study/NCT05738486.
- Kisunla (donanemab-azbt). Prescribing Information. Lilly
USA , LLC. - Data on File. Lilly
USA , LLC. DOF-DN-US-0029.
Refer to: | J.K. Wall; jkwall@lilly.com ; 317-433-5328 (Media) |
Joe Fletcher; jfletcher@lilly.com; 317-296-2884 (Investors) |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/modified-titration-of-donanemab-demonstrated-reduction-of-aria-e-in-early-symptomatic-alzheimers-disease-patients-in-phase-3b-study-302290351.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/modified-titration-of-donanemab-demonstrated-reduction-of-aria-e-in-early-symptomatic-alzheimers-disease-patients-in-phase-3b-study-302290351.html
SOURCE Eli Lilly and Company