LivaNova Unveils Essenz In-Line Blood Monitor with U.S. FDA 510(k) Clearance and CE Mark
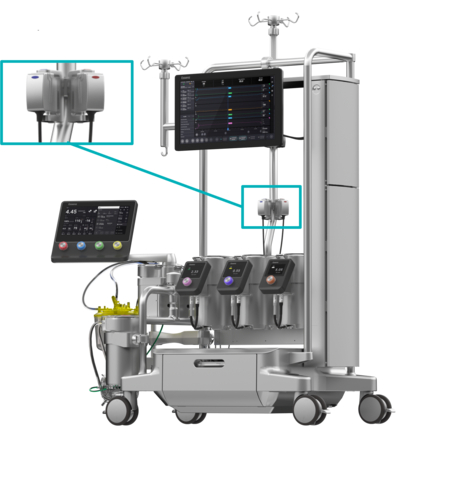
Essenz In-Line Blood Monitor shown with the Essenz Perfusion System (Photo: Business Wire)
Existing blood gas analyzers only reflect a patient’s clinical condition at the exact moment a sample is drawn, which can quickly change and become irrelevant.1,2 With the Essenz ILBM, perfusionists receive in-line continuous monitoring of the patient’s parameters for the duration of a procedure. This allows for the delivery of a patient-tailored approach to perfusion rooted in data-driven decisions.
“Dynamic conditions can rapidly change a patient’s blood parameters during a cardiopulmonary bypass procedure,” said Marco Dolci, LivaNova President, Cardiopulmonary. “The Essenz In-Line Blood Monitor provides continuous monitoring throughout a patient’s procedure. Access to accurate, real-time measurements directly from the Essenz Perfusion System allows for quick decisions and tailored care strategies to serve the patient.”
Powered by the proven B-Capta™ sensing technology, the Essenz ILBM is the only in-line blood monitoring system that works within Clinical Laboratory Improvement Amendments (CLIA) guidelines and provides parameter values in line with hospital blood gas analyzers, even prior to alignment.3 To enable accurate monitoring, the ILBM provides measured values for oxygen saturation, hematocrit, partial pressure of oxygen and temperature, rather than calculated values for these parameters.
In addition, Essenz ILBM requires no calibration to set device measurements, allowing the perfusionist to save time during device set up, especially in emergency cases. Arterial and venous parameters are automatically transferred to the Essenz™ Patient Monitor, supporting data-driven decision making and the implementation of goal-directed perfusion (GDP), a therapy effective in reducing the risk of acute kidney injury.4 The latest heart-lung machine software, version 1.3, integrates the ILBM with the Essenz Perfusion System and was developed to continually enhance the user experience.
Based on 50 years of trusted partnership, the Essenz Perfusion System was designed and developed in collaboration with more than 300 customers worldwide. It consists of a next-generation heart-lung machine (HLM), a patient monitor and accurate sensing technology that now includes the ILBM. The Essenz Perfusion System is currently available in
View the Essenz Perfusion System brand video here.
*Note: The Essenz HLM is not available for sale in all geographies. Visit the LivaNova website for important safety information.
References
- Ottens J. et all. Improving Cardiopulmonary Bypass: Does Continuous Blood Gas Monitoring Have a Role to Play? - JECT. 2010;42:191–198
- Trowbridge CC et al., The Effects of Continuous Blood Gas Monitoring During Cardiopulmonary Bypass: A Prospective, Randomized Study-Part II, The Journal of Extracorporeal Technology, 2000
- Perfusion, 2022, Vol. 0(0) 1–7, Marloes van Hoeven, Eddy Overdevest, Joyce Curvers, Henri van Heugten: A comparison of continuous blood gas monitors during cardiopulmonary bypass LivaNova B-Capta, Terumo CDI 500, spectrum medical M4
-
Goal-Directed Perfusion to reduce Acute Kidney Injury: A Randomized Trial Ranucci M. et al.
J Thorac Cardiovasc Surg. 2018 Nov; 156(5): 1918-1927.e2. doi.org/10.1016/j.jtcvs.2018.04.045
About LivaNova
LivaNova PLC is a global medical technology company built on nearly five decades of experience and a relentless commitment to provide hope for patients and their families through medical technologies, delivering life-changing improvements for both the Head and Heart. Headquartered in
Safe Harbor Statement
This news release contains “forward-looking statements” concerning the Company’s goals, beliefs, expectations, strategies, objectives, plans and underlying assumptions and other statements that are not necessarily based on historical facts. These statements include, but are not limited to, statements regarding the Essenz Perfusion System, Essenz HLM, the Essenz Patient Monitor and the Essenz ILBM. Actual events may differ materially from those indicated in our forward-looking statements as a result of various factors, including those factors set forth in Item 1A of the Company’s most recent Annual Report on Form 10-K, as supplemented by any risk factors contained in Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. LivaNova undertakes no obligation to update the information contained in this press release to reflect subsequently occurring events or circumstances.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230830793488/en/
LivaNova Investor Relations and Media Contacts
+1 281-895-2382
Briana Gotlin
Director, Investor Relations
InvestorRelations@livanova.com
Deanna Wilke
VP, Corporate Communications
Corporate.Communications@livanova.com
Source: LivaNova PLC







