U.S. Physicians Can Now Order Lucira Health Self-Administered, Lab Quality, COVID-19 Molecular Test for Home or Office
Lucira Health, Inc. (Nasdaq: LHDX), today announced that U.S. physicians and healthcare providers can now order Lucira COVID-19 All-In-One Test Kits online from lucirahealth.com. This U.S. made and manufactured product is the first FDA authorized, prescription, molecular diagnostic test for COVID-19 that can be self-administered in a physician’s office, or used by patients at home. Each single-use test kit costs
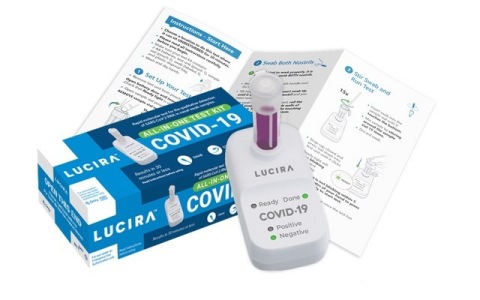
Lucira COVID-19 All-In-One Test Kits can be self-administered in a physician’s office, or used by patients at home. (Photo: Business Wire)
“Physicians in private practice like myself have been overwhelmed by patients suspected of having COVID-19. All of us want fast, accurate test results in minutes, not days. The Lucira test makes that possible,” said Dr. Neeraj Kochhar, M.D., a family medicine specialist in Los Gatos, California.
U.S. physicians who order the test can use it in their offices, or provide it to patients suspected of having COVID-19. It is the first molecular test kit that can be self-administered, and was designed and tested for use at home without requiring any telehealth or supervised assistance.
Featuring an easy-to-use ‘swab, stir and detect’ design, clinical trials showed
High sensitivity molecular tests like Lucira’s are significantly more sensitive than “rapid” antigen tests which do not work as well at very low viral loads. The result is they can miss active COVID-19 infections. Molecular tests such as PCR (polymerase chain reaction) and Lucira’s test are considered diagnostically definitive, unlike antigen tests which can require confirmatory molecular testing.
In a Community Trial setting, Lucira test results were compared with the Hologic Panther Fusion, which is considered one of the current market-leading molecular assays in an FDA published study because of its low Limit of Detection. The comparative positive results agreed
Portable, accurate test
Lucira’s COVID-19 All-In-One Test Kit fits in the palm of a hand, extracts genetic material from the virus and amplifies it similar to PCR lab tests.
Each Lucira test kit contains everything needed to run one COVID-19 test: Users get the test device, two AA batteries, sample vial, swab and simple instructions. The batteries are inserted in the device and the sample vial is placed in the test unit. Next, the user opens the test swab packet and rotates the swab in each nostril five times. The swab is then stirred in the sample vial, and then pressed down in the test unit to start the test. The “ready” light will blink until a “positive” or “negative” green light is illuminated within 30 minutes. For guidance on care and public health reporting, patients will contact or report their test results to the healthcare provider that prescribed the test.
Lucira Health
Lucira Health a medical technology company focused on the development and commercialization of transformative and innovative infectious disease test kits. Lucira has developed a testing platform that produces centralized-laboratory-accurate molecular testing in a single-use and consumer-friendly test kit that is powered by two AA batteries and fits in the palm of a hand. Lucira designed its test kits to provide accurate, reliable and on-the-spot molecular test results anywhere and at any time. The LUCIRA COVID-19 All-In-One Test Kit is designed to provide a clinically relevant COVID-19 result within 30 minutes from sample collection.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210211005343/en/







