Labcorp Expands Sexually Transmitted Infection (STI) Test Offerings with Rapid Syphilis Test
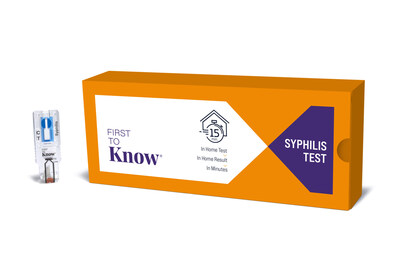
Labcorp (NYSE: LH) has expanded its STI testing portfolio with the First to Know® Syphilis Test, the first FDA-authorized over-the-counter blood test for syphilis. As the exclusive distributor for NOWDiagnostics (NOWDx) in the U.S., Labcorp will offer this rapid test to healthcare providers nationwide by the end of 2024 and to patients through Labcorp OnDemand in 2025.
The test provides results in 15 minutes using a single drop of blood from a fingerstick. This expansion aims to combat the rising syphilis cases in the U.S., which have increased by 80% between 2018 and 2022, reaching over 207,000 reported cases in 2022. Labcorp's syphilis testing volume has more than doubled in the past decade, exceeding 5.5 million tests annually.
Labcorp (NYSE: LH) ha ampliato il suo portafoglio di test per le IST con il First to Know® Syphilis Test, il primo test del sangue da banco autorizzato dalla FDA per la sifilide. In qualità di distributore esclusivo per NOWDiagnostics (NOWDx) negli Stati Uniti, Labcorp offrirà questo test rapido ai fornitori di servizi sanitari a livello nazionale entro la fine del 2024 e ai pazienti attraverso Labcorp OnDemand nel 2025.
Il test fornisce risultati in 15 minuti utilizzando una sola goccia di sangue prelevata dal dito. Questa espansione mira a combattere l'aumento dei casi di sifilide negli Stati Uniti, che sono aumentati del 80% tra il 2018 e il 2022, raggiungendo oltre 207.000 casi segnalati nel 2022. Il volume di test per la sifilide di Labcorp è più che raddoppiato nell'ultimo decennio, superando 5,5 milioni di test all'anno.
Labcorp (NYSE: LH) ha ampliado su portafolio de pruebas de ITS con la First to Know® Syphilis Test, la primera prueba de sangre de venta libre autorizada por la FDA para la sífilis. Como distribuidor exclusivo de NOWDiagnostics (NOWDx) en los EE. UU., Labcorp ofrecerá esta prueba rápida a los proveedores de atención médica en todo el país para finales de 2024 y a los pacientes a través de Labcorp OnDemand en 2025.
La prueba proporciona resultados en 15 minutos utilizando una sola gota de sangre de un pinchazo en el dedo. Esta expansión tiene como objetivo combatir el aumento de los casos de sífilis en los EE. UU., que han aumentado un 80% entre 2018 y 2022, alcanzando más de 207,000 casos reportados en 2022. El volumen de pruebas de sífilis de Labcorp se ha más que duplicado en la última década, superando 5.5 millones de pruebas al año.
랩코프(Labcorp)(NYSE: LH)는 First to Know® Syphilis Test로 성병 검사 포트폴리오를 확장했습니다. 이는 FDA에서 승인한 최초의 비처방 혈액 검사입니다. 랩코프는 미국 내 NOWDiagnostics(NOWDx)의 독점 유통업체로, 2024년 말까지 의료 제공자에게 이 신속 검사를 제공하고, 2025년부터는 Labcorp OnDemand를 통해 환자에게 제공할 예정입니다.
이 검사는 손가락에서 채혈한 단 한 방울의 혈액으로 15분 만에 결과를 제공합니다. 이 확장의 목적은 2018년과 2022년 사이에 80% 증가한 미국 내 매독 사례의 증가에 대응하기 위함입니다. 2022년에는 2만 7000건 이상의 신고된 사례에 도달했습니다. 랩코프의 매독 검사량은 지난 10년 이상 두 배 이상 증가하여 연간 550만 건을 초과했습니다.
Labcorp (NYSE: LH) a élargi son portefeuille de tests IST avec le First to Know® Syphilis Test, le premier test sanguin en vente libre autorisé par la FDA pour la syphilis. En tant que distributeur exclusif de NOWDiagnostics (NOWDx) aux États-Unis, Labcorp proposera ce test rapide aux professionnels de santé à l'échelle nationale d'ici fin 2024 et aux patients via Labcorp OnDemand en 2025.
Le test fournit des résultats en 15 minutes à partir d'une seule goutte de sang prélevée d'une piqûre au doigt. Cette extension vise à lutter contre l'augmentation des cas de syphilis aux États-Unis, qui ont augmenté de 80% entre 2018 et 2022, atteignant plus de 207 000 cas signalés en 2022. Le volume de tests de syphilis de Labcorp a plus que doublé au cours de la dernière décennie, dépassant 5,5 millions de tests par an.
Labcorp (NYSE: LH) hat sein STI-Testportfolio mit dem First to Know® Syphilis Test erweitert, dem ersten von der FDA autorisierten Bluttest für Syphilis, der rezeptfrei erhältlich ist. Als exklusiver Distributor für NOWDiagnostics (NOWDx) in den USA wird Labcorp diesen Schnelltest bis Ende 2024 bundesweit Gesundheitsdienstleistern anbieten und 2025 auch Patienten über Labcorp OnDemand zur Verfügung stellen.
Der Test liefert Ergebnisse in 15 Minuten mit einem einzigen Tropfen Blut aus einem Fingerstich. Diese Erweiterung zielt darauf ab, den Anstieg der Syphilisfälle in den USA zu bekämpfen, die zwischen 2018 und 2022 um 80% gestiegen sind und 2022 über 207.000 gemeldete Fälle erreicht haben. Das Testvolumen von Labcorp für Syphilis hat sich im letzten Jahrzehnt mehr als verdoppelt und übersteigt 5,5 Millionen Tests pro Jahr.
- Exclusive distribution agreement with NOWDiagnostics for the First to Know® Syphilis Test
- Expansion of STI testing portfolio with FDA-authorized rapid syphilis test
- Planned availability to healthcare providers by end of 2024 and patients through Labcorp OnDemand in 2025
- Syphilis testing volume has more than doubled over the past decade, exceeding 5.5 million tests annually
- None.
Insights
The introduction of the First to Know® Syphilis Test by Labcorp represents a significant advancement in STI diagnostics. This FDA-authorized over-the-counter blood test offers rapid results in just 15 minutes, potentially revolutionizing syphilis detection. The test's versatility for both clinical and home use could dramatically increase testing accessibility and frequency.
The 80% surge in U.S. syphilis cases between 2018 and 2022 underscores the urgent need for expanded testing options. Labcorp's exclusive distribution agreement with NOWDiagnostics positions them at the forefront of addressing this public health crisis. The planned availability to providers by end of 2024 and to patients through Labcorp OnDemand in 2025 could significantly impact early detection rates.
However, it's important to note that this test is not a standalone diagnostic tool. The requirement for additional confirmatory testing aligns with CDC guidelines but may add complexity to the testing process. The test's impact on Labcorp's revenue streams and market position in STI diagnostics could be substantial, given their current annual volume of 5.5 million syphilis tests.
Labcorp's exclusive distribution agreement for the First to Know® Syphilis Test presents a significant market opportunity. With the U.S. syphilis testing market expanding rapidly, this move could solidify Labcorp's position as a leader in STI diagnostics. The company's established infrastructure and wide reach in the healthcare sector provide a strong foundation for successful distribution and adoption of this new test.
The planned rollout strategy, first to healthcare providers and then directly to consumers, is astute. It allows for professional validation and familiarization before entering the potentially lucrative direct-to-consumer market. This approach could drive substantial revenue growth, especially considering Labcorp's existing high volume of syphilis tests.
Investors should note the potential for this product to diversify Labcorp's revenue streams and enhance its competitive edge in the diagnostics market. However, the success will depend on factors such as pricing strategy, marketing effectiveness and the rate of adoption by healthcare providers and consumers. The move aligns well with the growing trend towards personalized and accessible healthcare solutions.
Labcorp to serve as the exclusive NOWDiagnostics (NOWDx) distributor for clinical testing in the
Labcorp will serve as the exclusive distributor of the First to Know Syphilis Test to providers in healthcare settings nationwide, through an agreement with over-the-counter and point-of-care diagnostic test developer NOWDiagnostics (NOWDx). This new offering reflects Labcorp's commitment to expanding provider and patient access to fast and reliable syphilis tests in an effort to help combat the syphilis epidemic.
"The recent rise in syphilis cases highlights a critical gap in testing and treatment across the country," said Dr. Brian Caveney, Labcorp's chief medical and scientific officer. "This test is another tool in a provider's arsenal as they face this serious public health emergency. Our agreement with NOWDx reflects our commitment to providing healthcare providers and patients across the country with access to tests that deliver fast and reliable results that empower them to make informed decisions about their health."
Labcorp will leverage its scale as one of the largest diagnostics laboratories in the world to facilitate widespread availability of the First to Know Syphilis Test, which provides a result in 15 minutes with as little as a single drop of blood collected through a fingerstick. Physicians can utilize the test in clinical settings or give it to a patient to conduct in the comfort of their own home. Labcorp plans to make the test available to providers by the end of 2024 and directly to patients through Labcorp OnDemand in 2025.
"Labcorp's strong infrastructure and commitment to advancing diagnostic technology and making testing widely accessible make it the ideal partner to help expand access to our tests and tackle critical public health issues, such as the rise in syphilis cases," said Robert Weigle, CEO of NOWDx. "Together, we aim to ensure our cutting-edge, user-friendly tests are within reach for more patients and healthcare providers, ultimately enhancing early identification and treatment outcomes."
As the FDA has noted, results from the First to Know Syphilis Test alone are not sufficient to diagnose syphilis infection and should be followed by additional testing to confirm a diagnosis of syphilis.
Labcorp currently offers treponemal and non-treponemal assays recommended by the Centers for Disease Control and Prevention (CDC) for the screening and diagnosis of syphilis. The company has seen syphilis testing volume more than double over the past decade, exceeding 5.5 million tests annually.
For more information, contact diagnosticsales@labcorp.com.
About Labcorp
Labcorp (NYSE: LH) is a global leader of innovative and comprehensive laboratory services that helps doctors, hospitals, pharmaceutical companies, researchers and patients make clear and confident decisions. We provide insights and advance science to improve health and improve lives through our unparalleled diagnostics and drug development laboratory capabilities. The company's more than 67,000 employees serve clients in approximately 100 countries, provided support for
About NOWDiagnostics (NOWDx)
NOWDx develops and manufactures over-the-counter (OTC) and point-of-care (POC) diagnostic tests. Its patented approach enables virtually any immunological assay to be accurately performed onsite in one step using a small amount of capillary blood, yielding results in minutes. With over 75 patents issued and pending, NOWDx's First To Know® and ADEXUSDx® product lines are available in markets worldwide. Founded in 2013, with headquarters and manufacturing in
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/labcorp-expands-sexually-transmitted-infection-sti-test-offerings-with-rapid-syphilis-test-302272890.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/labcorp-expands-sexually-transmitted-infection-sti-test-offerings-with-rapid-syphilis-test-302272890.html
SOURCE Labcorp