Lexaria's DehydraTECH-tirzepatide Oral Capsules Achieve Comparable Levels in Bloodstream as Eli Lilly’s Injectable Zepbound(R)
Lexaria Bioscience (NASDAQ:LEXX) announced positive results from Human Study #3 comparing oral DehydraTECH-tirzepatide capsules to injectable Zepbound®. The study showed that oral capsules achieved comparable blood concentration levels to injections by the study's end.
Key findings from the 8-day study of 19 participants revealed that while injected Zepbound® typically peaked on Day 2 and declined, DehydraTECH-tirzepatide levels increased steadily daily. The oral version demonstrated:
- 47% fewer adverse events (20 vs 38)
- 54% reduction in gastrointestinal side effects
- Similar blood glucose reduction and insulin increase by Day 8
The company is now conducting a 12-week Phase 1b registrational study in Australia, starting at 20mg/day for 4 weeks and escalating to 40mg/day for the remaining 8 weeks. This extended study aims to evaluate steady-state blood levels and potentially demonstrate increased delivery and efficacy.
Lexaria Bioscience (NASDAQ:LEXX) ha annunciato risultati positivi dallo Studio Umano #3 che confronta le capsule orali di DehydraTECH-tirzepatide con Zepbound® iniettabile. Lo studio ha mostrato che le capsule orali hanno raggiunto livelli di concentrazione nel sangue comparabili a quelli delle iniezioni entro la fine dello studio.
I principali risultati dello studio di 8 giorni condotto su 19 partecipanti hanno rivelato che, mentre Zepbound® iniettato raggiungeva tipicamente il picco il Giorno 2 e poi diminuiva, i livelli di DehydraTECH-tirzepatide aumentavano costantemente ogni giorno. La versione orale ha dimostrato:
- 47% in meno di eventi avversi (20 contro 38)
- 54% di riduzione degli effetti collaterali gastrointestinali
- Riduzione simile della glicemia e aumento dell'insulina entro il Giorno 8
L'azienda sta ora conducendo uno studio registrativo di Fase 1b di 12 settimane in Australia, iniziando con 20mg/giorno per 4 settimane e aumentando a 40mg/giorno per le restanti 8 settimane. Questo studio esteso mira a valutare i livelli ematici a stato stazionario e potenzialmente dimostrare un aumento della somministrazione e dell'efficacia.
Lexaria Bioscience (NASDAQ:LEXX) anunció resultados positivos del Estudio Humano #3 que compara las cápsulas orales de DehydraTECH-tirzepatide con Zepbound® inyectable. El estudio mostró que las cápsulas orales lograron niveles de concentración en sangre comparables a las inyecciones al final del estudio.
Los hallazgos clave del estudio de 8 días con 19 participantes revelaron que, mientras que Zepbound® inyectado generalmente alcanzaba su punto máximo en el Día 2 y luego disminuía, los niveles de DehydraTECH-tirzepatide aumentaban constantemente cada día. La versión oral demostró:
- 47% menos eventos adversos (20 frente a 38)
- 54% de reducción en efectos secundarios gastrointestinales
- Reducción similar de glucosa en sangre y aumento de insulina para el Día 8
La empresa está llevando a cabo ahora un estudio registrativo de Fase 1b de 12 semanas en Australia, comenzando con 20mg/día durante 4 semanas y aumentando a 40mg/día durante las 8 semanas restantes. Este estudio ampliado tiene como objetivo evaluar los niveles de sangre en estado estable y potencialmente demostrar un aumento en la entrega y eficacia.
Lexaria Bioscience (NASDAQ:LEXX)는 주사형 Zepbound®와 비교하여 경구용 DehydraTECH-tirzepatide 캡슐의 긍정적인 결과를 발표했습니다. 연구 결과, 경구용 캡슐이 연구 종료 시점에서 주사와 유사한 혈중 농도 수준을 달성한 것으로 나타났습니다.
19명의 참가자를 대상으로 한 8일간의 연구에서 주요 결과는 주사형 Zepbound®가 일반적으로 2일째에 정점을 찍고 감소하는 반면, DehydraTECH-tirzepatide의 수준은 매일 꾸준히 증가했다는 것입니다. 경구 버전은 다음과 같은 결과를 보여주었습니다:
- 부작용 47% 감소(20 대 38)
- 위장관 부작용 54% 감소
- 8일째에 유사한 혈당 감소 및 인슐린 증가
회사는 현재 호주에서 12주 동안의 1b상 등록 연구를 진행 중이며, 4주 동안 20mg/일로 시작하여 나머지 8주 동안 40mg/일로 증량할 예정입니다. 이 확장된 연구는 안정적인 혈중 수준을 평가하고 잠재적으로 전달 및 효능의 증가를 입증하는 것을 목표로 하고 있습니다.
Lexaria Bioscience (NASDAQ:LEXX) a annoncé des résultats positifs de l'Étude Humaine #3 comparant les capsules orales de DehydraTECH-tirzepatide aux injections de Zepbound®. L'étude a montré que les capsules orales atteignaient des niveaux de concentration sanguine comparables à ceux des injections à la fin de l'étude.
Les résultats clés de l'étude de 8 jours menée sur 19 participants ont révélé que, tandis que Zepbound® injecté atteignait généralement un pic au Jour 2 puis diminuait, les niveaux de DehydraTECH-tirzepatide augmentaient régulièrement chaque jour. La version orale a démontré:
- 47% d'événements indésirables en moins (20 contre 38)
- 54% de réduction des effets secondaires gastro-intestinaux
- Réduction similaire de la glycémie et augmentation de l'insuline au Jour 8
L'entreprise mène maintenant une étude d'enregistrement de Phase 1b de 12 semaines en Australie, commençant à 20mg/jour pendant 4 semaines et augmentant à 40mg/jour pour les 8 semaines restantes. Cette étude prolongée vise à évaluer les niveaux sanguins à l'état d'équilibre et à démontrer potentiellement une augmentation de la délivrance et de l'efficacité.
Lexaria Bioscience (NASDAQ:LEXX) hat positive Ergebnisse aus der Humanstudie #3 bekannt gegeben, die orale DehydraTECH-tirzepatide-Kapseln mit injizierbarem Zepbound® vergleicht. Die Studie zeigte, dass die oralen Kapseln am Ende der Studie vergleichbare Blutkonzentrationsniveaus wie die Injektionen erreichten.
Wichtige Ergebnisse der 8-tägigen Studie mit 19 Teilnehmern zeigten, dass während injiziertes Zepbound® typischerweise am Tag 2 seinen Höhepunkt erreichte und dann abnahm, die Werte von DehydraTECH-tirzepatide täglich stetig zunahmen. Die orale Version zeigte:
- 47% weniger unerwünschte Ereignisse (20 gegenüber 38)
- 54% Reduzierung gastrointestinaler Nebenwirkungen
- Ähnliche Senkung des Blutzuckerspiegels und Anstieg des Insulins bis Tag 8
Das Unternehmen führt nun eine 12-wöchige Phase-1b-Registrierungsstudie in Australien durch, die mit 20mg/Tag für 4 Wochen beginnt und auf 40mg/Tag für die verbleibenden 8 Wochen erhöht wird. Diese erweiterte Studie zielt darauf ab, stabile Blutspiegel zu bewerten und möglicherweise eine erhöhte Verabreichung und Wirksamkeit zu demonstrieren.
- Oral DehydraTECH-tirzepatide achieved comparable blood concentration levels to injectable Zepbound
- 47% reduction in adverse events compared to injectable version
- 54% decrease in gastrointestinal side effects
- Similar efficacy in blood glucose reduction and insulin increase
- More consistent daily accumulation compared to weekly injection peaks and valleys
- Peak blood delivery levels were generally lower than injectable version
- Requires daily dosing compared to weekly injection of Zepbound
Insights
Lexaria's DehydraTECH oral tirzepatide delivery system has demonstrated unexpectedly strong clinical results that could represent a significant advancement in GLP-1 medication administration. The company's Human Study #3 showed their oral capsules achieved comparable blood concentration levels to Eli Lilly's injectable Zepbound by study end, with 47% fewer adverse events and similar glycemic performance.
What makes this particularly noteworthy is that tirzepatide (Zepbound/Mounjaro) is only available as an injection currently, with no oral formulation on the market. The technical achievement of creating an oral delivery system that maintains efficacy while reducing side effects addresses a significant market need, as injection administration remains a barrier for many potential GLP-1 users.
The pharmacokinetic profile of DehydraTECH-tirzepatide shows a more gradual, consistent accumulation pattern compared to the injection's peak-and-decline profile. This more stable delivery could potentially improve tolerability while maintaining therapeutic effect. The company's advance to a 12-week Phase 1b registrational study with dose escalation (from 20mg/day to 40mg/day) demonstrates confidence in the technology's capabilities.
For context, Novo Nordisk paid
More consistent accumulation demonstrated in bloodstream over a one-week duration with once-daily DehydraTECH-tirzepatide oral capsules as compared to once-weekly injection of Zepbound®
As previously announced, oral DehydraTECH-tirzepatide also reduced adverse events by
47% compared to injected Zepbound®Lexaria's oral capsules worthy of expanded investigation as a viable alternative to injected tirzepatide.
KELOWNA, BC / ACCESS Newswire / March 18, 2025 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms, is pleased to announce positive pharmacokinetic ("PK") results from Human Study #3 or GLP-1-H24-3 (the "Study"), comparing an oral version of DehydraTECH-processed Zepbound® ("DehydraTECH-tirzepatide") to conventional injected Zepbound®.
Zepbound® is currently only available as a once-weekly injection for weight loss. It is not sold by Eli Lilly in any oral format. Lexaria's Study was designed to discover whether the active drug within Zepbound® - tirzepatide - could be administered using Lexaria's patented DehydraTECH drug delivery technology via simple oral capsules in order to deliver a useful quantity of tirzepatide into the human bloodstream, as well as provide a viable alternative to disliked injections. The results were unexpectedly positive, showing that orally delivered DehydraTECH-tirzepatide reached roughly equal end of Study blood-concentration levels as the injected drug.
During the 8-day Study, data was successfully collected from 10 people who were dosed with a single weekly injection of Zepbound®, and from 9 people who were dosed daily over the same one-week duration with DehydraTECH-tirzepatide capsules.
In general, the peak levels of blood delivery of the injected Zepbound® were, for the most part, higher than that of the DehydraTECH-tirzepatide, but not in all cases. The injected Zepbound® typically reached a peak level within blood on the 2nd day of the Study and subsequently declined. Conversely, the DehydraTECH-tirzepatide blood levels increased steadily and more consistently each day of the Study, avoided any abrupt peaks or declines, and were generally still rising on Day 8, the final day of the Study. Of those receiving the Zepbound® injection, 8 of 10 people (
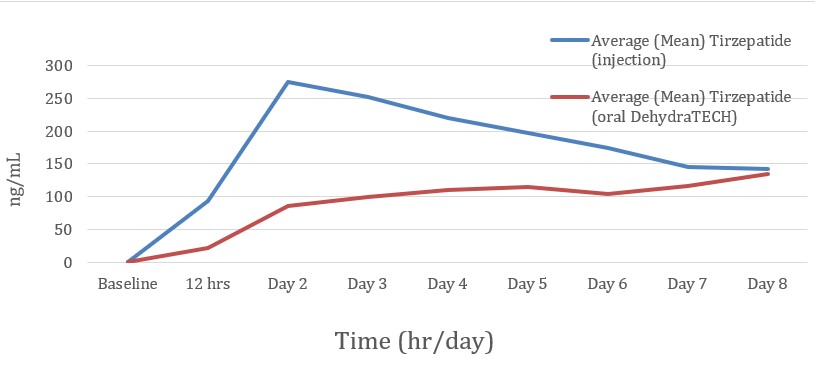
As announced on January 14, 2025, during the Study DehydraTECH-tirzepatide also evidenced reduced side effects, while achieving comparable concluding glycemic performance indicators. The injected Zepbound® produced a total of 38 adverse events during the Study, whereas the oral DehydraTECH-tirzepatide produced only 20, or
"Lexaria's first-ever study of oral DehydraTECH-tirzepatide has far exceeded our expectations," said Richard Christopher, CEO of Lexaria. "We have succeeded in demonstrating all 3 of our main objectives; reduced side effects with similar efficacy and similar blood-delivery levels as compared to injected tirzepatide by the end of the Study. Our ongoing 12-week study in Australia is well positioned to further evaluate the effectiveness of DehydraTECH over an extended dosing duration and potentially establish Lexaria as a global player in oral delivery within the fast-growing GLP-1 weight loss and diabetes control markets."
All the results of this study - reduced side effects, comparable end of Study blood sugar control and measured drug in bloodstream - are extremely encouraging and support our decision to further evaluate DehydraTECH-tirzepatide in our ongoing Australian Phase 1b registrational study (GLP-1-H24-4). In that 12-week study, DehydraTECH-tirzepatide will be dosed at the same 20mg/day level utilized in Human Study #3 for the initial 4 weeks of treatment, escalating further to 40mg/day over the last 8 weeks of treatment, thereby allowing Lexaria to potentially demonstrate further increased delivery and efficacy relative to the present Study.
Lexaria looks forward to the opportunity to assess the very important steady-state blood levels which DehydraTECH-tirzepatide administered via its oral capsules will achieve over an extended dosing duration in study GLP-1-H24-4 as they relate to published figures for sustained injectable tirzepatide dosing, given the fact that blood levels from DehydraTECH-tirzepatide witnessed in the present Study were continuing to ascend at Day 8.
There is no oral version of tirzepatide sold in the world today, as it is administered only by injection (Zepbound® and Mounjaro®). Lexaria has previously completed other research with oral DehydraTECH-processed semaglutide, sold by Novo Nordisk®, which is the only GLP-1 drug that is currently available as both an oral (Rybelsus®) and an injectable (Ozempic® and Wegovy®). This research yielded similar findings wherein Lexaria's DehydraTECH-processed semaglutide evidenced certain improvements in oral delivery compared to Rybelsus®. Lexaria believes that an effective oral version of tirzepatide with fewer adverse events than the current injectable versions, could be highly valued.
As noted, Rybelsus® is the only orally delivered GLP-1 drug on the market today. Rybelsus® uses a proprietary drug delivery technology called salcaprozate sodium ("SNAC"), that Novo Nordisk paid US
About the Study
Many design characteristics of the Study, also referred to as Study GLP-1-H24-3, are similar to Lexaria's initial GLP-1 human pilot study #1, investigating the dual agonist GLP-1/GIP drug tirzepatide in this Study instead of the GLP-1 agonist semaglutide from human pilot study #1. The DehydraTECH-tirzepatide test articles were compound formulated using Zepbound®, strictly for research purposes, and dosed orally to the subjects under fasted conditions. The Study was designed to measure tolerability and side effects, blood levels of tirzepatide, and blood glucose and insulin levels. The DehydraTECH-tirzepatide composition was formulated at a strength of 20 mg tirzepatide administered orally daily for seven days followed through to the end of the eighth day post-dosing. The Zepbound® formulation had a strength of 2.5 mg tirzepatide administered once via injection with the subject monitored over the same eight-day total duration. Blood samples were taken multiple times during the first 12 hours post dosing on the first day of each treatment phase, with single blood samples taken daily thereafter through to a final blood draw taken 24 hours after the end of dosing (i.e., on the eighth day of the Study); and, all subjects were dosed under fasted conditions with a standardized meal fed to the test subjects at a point in time after dosing. Subjects were dosed with each test article following a randomized, cross over study design across two study phases, separated by a 4-6 week washout duration.
About Lexaria Bioscience Corp. & DehydraTECH
DehydraTECH™ is Lexaria's patented drug delivery formulation and processing platform technology which improves the way a wide variety of drugs enter the bloodstream, always through oral delivery. DehydraTECH has repeatedly evidenced the ability to increase bio-absorption, reduce side-effects, and deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 48 patents granted and additional patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View the original press release on ACCESS Newswire






