Lexaria Releases Strategic Letter from the Outgoing CEO
Rhea-AI Summary
Lexaria Bioscience Corp. (NASDAQ:LEXX) announces a strategic update as CEO Chris Bunka steps down. Key points include:
- New focus on pharmaceutical studies in cardiometabolic and GLP-1 sectors
- Appointment of Richard Christopher as new CEO with pharma/biotech operations experience
- FDA clearance for Phase 1b hypertension study using DehydraTECH-CBD
- Over $8.5 million raised in 2024 through equity raises and warrant exercises
- Market cap growth from $5 million in June 2023 to $60 million
- Ongoing GLP-1 studies with promising results
- Plans for two Phase 1 studies in 2025
- Material Transfer Agreement with a pharmaceutical company
Bunka will continue as Executive Strategic Advisor and Chairman of the Board.
Positive
- FDA clearance received for Phase 1b hypertension trial using DehydraTECH-CBD
- Over $8.5 million raised in 2024 through equity raises and warrant exercises
- Market capitalization grew from $5 million to $60 million in 15 months
- Positive results in GLP-1 R&D program, demonstrating potential superiority in oral semaglutide and liraglutide
- Material Transfer Agreement signed with a pharmaceutical company
- Plans for two Phase 1 studies in 2025: hypertension and GLP-1 diabetes/weight-loss
Negative
- Additional funding required to launch the Phase 1b hypertension study
- Delay in starting the hypertension study, now likely to begin in 2025
- CEO transition may cause temporary operational disruption
News Market Reaction 1 Alert
On the day this news was published, LEXX declined 4.07%, reflecting a moderate negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
KELOWNA, BC / ACCESSWIRE / September 5, 2024 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms is pleased to provide this letter from outgoing Chief Executive Officer ("CEO") Chris Bunka as a strategic update to all stakeholders.
CEO LETTER TO STAKEHOLDERS
It is with mixed emotions and a heavy heart that I write my final strategic letter to my Lexaria family. Lexaria has been "home" for 16 years, and, similar to raising a family, there have been difficult times that test your resolve, and many other times of incredible joy and pride.
Many people will focus on the "why". There is just one, fairly simple reason: Lexaria has arrived! We have finally found a sector that desperately needs our technology and we are pursuing our commercial opportunities vigorously; this includes our recently announced entrance into a Material Transfer Agreement with a pharmaceutical company. With our clear new focus, the need to pivot - one of my particular skillsets - is no longer necessary. Lexaria is now focused on pharmaceutical studies in the cardiometabolic and GLP-1 sectors; on operations; and, on expected industry collaborations.
Given the new focus, we need a steely-eyed CEO with years of pharma/biotech operations experience - something I do not possess. Under most of my tenure our strategy was one of exploration. Our strategy today has evolved to one of execution. Today we are well known to the investment banking and investor communities, and we are increasingly known to the pharmaceutical industry. Our immediate tasks are no longer primarily focused on discovery - today we focus on execution. Our new CEO, Richard Christopher, has years of experience running operations and facilities, dealing with the FDA and other regulators, and loads of public market and financing experience. He also has the "fire in the belly" that cannot be taught.
Lexaria has graduated! It is time for our exciting new chapters to unfold.
CAPITAL MARKETS and SHAREHOLDERS
We've had a truly exciting first eight months of 2024!
We filed our IND with the FDA and received our clearance letter to proceed with our Phase 1b hypertension study. Subject to financing, that study is likely to begin in 2025.
We've raised over
$8.5 million during 2024, through equity raises and warrant exercises.We launched multiple studies in GLP-1 and have recently started reporting results.
Our market capitalization has grown from roughly
$5 million in June of 2023 to approximately$60 million today.
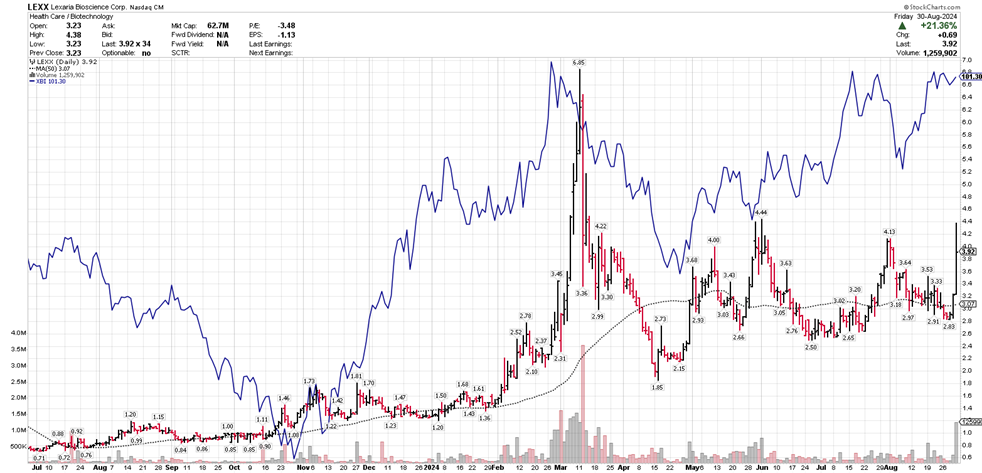
Lexaria has experienced strong trading volume relative to our still-small share capital structure, and we have many new shareholders since we've announced our focus on the GLP-1 sector. I'm confident that we have the strongest and smartest shareholder base ever. It has been my pleasure to serve you during my tenure, and many of you know that I have always placed you first. I truly believe that Lexaria's new CEO, Richard Christopher, will be able to serve your needs during this next exciting phase of our growth, better than I.
Shorters and distorters are an unfortunate part of the market environment and roughly equally as useful as buzzards - they serve a purpose and feed off of emotional reactivity. But to all of you, I would say; never believe anything you read on chat boards - the entire concept of anonymous posting begs for abuse. For our thousands of shareholders, be sure you get your news directly from the Company and from our regulatory filings. I encourage everyone to phone or email George Jurcic "our internal IR guy" if you need explanations or require additional information (contact information below).
Phase 1 Studies
On March 1, 2024, we received clearance from the FDA for our Phase 1b hypertension trial utilizing DehydraTECH-CBD ("DHT-CBD"). Since then, we have been busy complying with other FDA requests for additional information; have made some design changes to the study and are also obtaining fresh quotes from service providers to conduct the study. We will be opportunistic in raising additional funds that will be required to launch that study, as well as to manufacture fresh DHT-CBD test articles as we get closer to a launch date. While we still do not have any firm dates, it seems highly likely that this registered trial may begin in the first half of 2025.
We meanwhile have created a new wholly-owned Australian subsidiary which has been very aggressive with our GLP-1 study investigations, and with our CRO, is currently working on our Australian-registered Phase 1b DehydraTECH-GLP-1 diabetes and weight-loss study to be conducted over 12 weeks of dosing. We have sufficient funds on hand to conduct this study. We are preparing for manufacturing of the drug product which should be completed within 100 days. Clinical site investigations are ongoing and Lexaria will have a more fulsome update on the progress of this study, soon.
So, it appears we are likely to have TWO Phase 1 studies running in 2025: quite an achievement for a company with a ~
R&D
Lexaria has been generating a lot of data from our ongoing GLP-1 studies, and the Company expects to do so for most of the rest of 2024 and 2025. I'm not intending to update each study here: review our recent press releases for more detailed information and watch for more upcoming news.
A couple of general points: Lexaria never expected to report double-digit percentage weight loss numbers from our current rodent study. If any of you had that expectation, I'm sorry to inform you that isn't realistic. Rodents will often eat themselves to a morbid state if given the opportunity to do so with unlimited food such as we offered during our ongoing rodent study. In this type of study, simply not gaining any additional weight can be considered something of a win.
Instead, we hoped the data would guide us in other pursuits such as deciding on formulations for our upcoming 12-week human study. It also is helpful to our imminent decisions to design and execute additional human pilot study work, and additional directional animal work. Stay tuned!
We have incredibly positive results accumulating utilizing DHT-CBD, and in our current animal study we have one specialized DHT-CBD formulation outperforming in weight loss applications, and another DHT-CBD formulation outperforming in blood sugar control. This is unexpectedly bountiful and strongly affirms our prior years of research and investigation into DHT-CBD.
As all you athletes know, you train and prepare for success by focusing on success - you repeat those things that deliver results. Lexaria is the same: we follow our successful data and look for ways to build commercial success from positive R&D data. I firmly believe that our recent success in GLP-1 R&D is going to manifest into commercial success in 2025. And yes, I'm aware that my earlier timelines have sometimes been too aggressive - but I'm also aware of the ripples that our recent work has created within the pharma industry leading, for example, to our recently announced Material Transfer Agreement with a pharmaceutical company. We are focused on success and will not rest until we have achieved both R&D and commercial success.
SUMMARY
Lexaria is enjoying considerable success in our GLP-1 R&D program, with results already demonstrating interesting areas of superiority including DehydraTECH-processed oral semaglutide and liraglutide, as well as DHT-CBD. The Company has also made great progress with its DHT-CBD in the hypertension space as an area of great promise for expanded registered clinical testing expected through 2025. Lexaria has stated publicly that we've caught the attention of "Big Pharma" and our ongoing discussions and relationships could provide for increased activity in the months and quarters to come.
It is because of the combination of events that include our successful money-raising, our R&D success, our new relationship with one pharmaceutical company and continued pursuit of others, and our confidence in our strategic direction for the foreseeable future, that I am using the opportunity to replace myself with someone with stronger skillsets than my own, in corporate operational areas that we've identified as necessary for our commercial success.
Indeed, it is my high level of confidence that commercial success is in our path, that endows me with the certainty to step aside for new leadership at Lexaria. I literally would work myself to death at Lexaria if I lacked that confidence - I can make no stronger a statement in my level of comfort in Lexaria than turning over the helm to a younger, more experienced executive ideally suited for the Company's evolution.
As I said in January, "I have been a shareholder of Lexaria for a very long time: I know how our shareholders feel as you watch our progress because I feel most of the same things you do. I've always tried to be straight-up with you even as we've juggled some daunting challenges. We have new shareholders… who have enthusiastically supported our most recent corporate strategies - to you I offer a special "thank you" for your support."
It is my honour to be part of Lexaria's past as well as contribute to its very bright future.
Chris Bunka
Mr. Bunka has been appointed as Executive Strategic Advisor to the management team where he will continue to provide support and advice to Lexaria. He also remains as Chairman of the Board of Directors of Lexaria and looks forward to providing more focused strategic leadership in that role.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream by promoting more effective oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, GLP-1 and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 46 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the Company relating to the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View the original press release on accesswire.com








