Pasithea Therapeutics Announces Positive Initial Safety, Tolerability, Pharmacokinetic (PK), and Preliminary Efficacy Data from its Phase 1 Clinical Trial of PAS-004 in Advanced Cancer
Pasithea Therapeutics (NASDAQ: KTTA) announced positive initial data from its Phase 1 clinical trial of PAS-004, a next-generation macrocyclic MEK inhibitor, for advanced cancer treatment. The trial, conducted at four U.S. sites, showed promising results in safety, tolerability, pharmacokinetics (PK), and preliminary efficacy.
Key findings include:
- No treatment-related adverse events or dose-limiting toxicities observed
- Long half-life of ~70 hours, supporting once-daily or less frequent oral dosing
- Constant target inhibition while avoiding peak plasma toxicities
- A patient with stage 3 colon cancer achieved prolonged stable disease
The company believes PAS-004 demonstrates a differentiated MEK inhibitor profile, potentially outperforming current inhibitors in safety, dosing frequency, and efficacy for treating neurofibromatosis type 1 (NF1) and other cancer indications.
Pasithea Therapeutics (NASDAQ: KTTA) ha annunciato dati iniziali positivi dal suo studio clinico di Fase 1 su PAS-004, un inibitore MEK macroiclico di nuova generazione, per il trattamento del cancro avanzato. Lo studio, condotto in quattro sedi negli Stati Uniti, ha mostrato risultati promettenti in termini di sicurezza, tollerabilità, farmacocinetica (PK) e efficacia preliminare.
Le principali scoperte includono:
- Nessun evento avverso correlato al trattamento o tossicità limitante per la dose osservati
- Emivita lunga di circa 70 ore, che supporta un dosaggio orale giornaliero o meno frequente
- Inibizione costante del bersaglio evitando le tossicità picco plasmatiche
- Un paziente con cancro del colon in fase 3 ha raggiunto una malattia stabile prolungata
L'azienda ritiene che PAS-004 dimostri un profilo di inibitore MEK differenziato, potenzialmente superiore agli inibitori attuali in termini di sicurezza, frequenza di dosaggio ed efficacia per il trattamento della neurofibromatosi di tipo 1 (NF1) e di altre indicazioni oncologiche.
Pasithea Therapeutics (NASDAQ: KTTA) anunció datos iniciales positivos de su ensayo clínico de Fase 1 de PAS-004, un inhibidor MEK macrocíclico de nueva generación, para el tratamiento del cáncer avanzado. El ensayo, llevado a cabo en cuatro sitios en EE. UU., mostró resultados prometedores en seguridad, tolerabilidad, farmacocinética (PK) y eficacia preliminar.
Los hallazgos clave incluyen:
- No se observaron eventos adversos relacionados con el tratamiento ni toxicidades limitantes de dosis
- Vida media larga de aproximadamente 70 horas, lo que apoya una dosificación oral diaria o menos frecuente
- Inhibición constante del objetivo evitando toxicidades pico en el plasma
- Un paciente con cáncer de colon en etapa 3 logró una enfermedad estable prolongada
La compañía cree que PAS-004 demuestra un perfil de inhibidor MEK diferenciado, con el potencial de superar a los inhibidores actuales en seguridad, frecuencia de dosificación y eficacia para tratar la neurofibromatosis tipo 1 (NF1) y otras indicaciones oncológicas.
Pasithea Therapeutics(NASDAQ: KTTA)는 고급 암 치료를 위한 차세대 매크로사이클 MEK 억제제 PAS-004의 1상 임상 시험에서 긍정적인 초기 데이터를 발표했습니다. 미국 내 4곳에서 실시된 이 임상 시험은 안전성, 내약성, 약동학(PK), 그리고 초기 효능에 대해 유망한 결과를 보여주었습니다.
주요 발견 사항은 다음과 같습니다:
- 치료와 관련된 부작용이나 용량 제한 독성이 관찰되지 않았습니다.
- 약 70시간의 긴 반감기는 하루 한번 또는 덜 자주 경구 투여할 수 있음을 지원합니다.
- 최대 혈장 독성을 피하면서 표적 억제의 지속성을 유지합니다.
- 3기 대장암을 앓고 있는 한 환자가 안정적인 병을 장기간 유지했습니다.
회사는 PAS-004가 차별화된 MEK 억제제 프로필을 보여주며, NF1(신경섬유종증 제1형) 및 기타 암 적응증 치료에 있어 현재의 억제제를 능가할 가능성이 있다고 믿고 있습니다.
Pasithea Therapeutics (NASDAQ: KTTA) a annoncé des données initiales positives de son essai clinique de Phase 1 sur PAS-004, un inhibiteur MEK macrocyclique de nouvelle génération, pour le traitement du cancer avancé. L'essai, mené dans quatre sites aux États-Unis, a montré des résultats prometteurs en matière de sécurité, de tolérabilité, de pharmacocinétique (PK) et d'efficacité préliminaire.
Les principales conclusions incluent :
- Aucun événement indésirable lié au traitement ou toxicités limitant la dose observés
- Une longue demi-vie d'environ 70 heures, soutenant une posologie orale quotidienne ou moins fréquente
- Inhibition constante de la cible tout en évitant les toxicités plasmatiques de pointe
- Un patient atteint d'un cancer du côlon au stade 3 a réussi à maintenir une maladie stable prolongée
La société estime que PAS-004 démontre un profil d'inhibiteur MEK différencié, pouvant potentiellement surperformer les inhibiteurs actuels en matière de sécurité, de fréquence de dosage et d'efficacité pour le traitement de la neurofibromatose de type 1 (NF1) et d'autres indications oncologiques.
Pasithea Therapeutics (NASDAQ: KTTA) hat positive erste Daten aus seiner Phase-1-Studie zu PAS-004, einem neuartigen makrozyklischen MEK-Inhibitor, für die Behandlung von fortgeschrittenen Krebserkrankungen bekannt gegeben. Die Studie, die an vier Standorten in den USA durchgeführt wurde, zeigte vielversprechende Ergebnisse in Bezug auf Sicherheit, Verträglichkeit, Pharmakokinetik (PK) und vorläufige Wirksamkeit.
Wichtige Ergebnisse umfassen:
- Keine behandlungsbedingten unerwünschten Ereignisse oder dosislimitierenden Toxizitäten beobachtet
- Lange Halbwertszeit von etwa 70 Stunden, die eine tägliche oder weniger häufige orale Dosierung unterstützt
- Ständige Zielinhibition bei gleichzeitiger Vermeidung von Spitzenplasma-Toxizitäten
- Ein Patient mit Stadium-3-Darmkrebs erreichte eine verlängerte stabile Erkrankung
Das Unternehmen ist der Meinung, dass PAS-004 ein differenziertes Profil als MEK-Inhibitor zeigt, das möglicherweise bestehende Inhibitoren in Bezug auf Sicherheit, Dosishäufigkeit und Wirksamkeit bei der Behandlung von Neurofibromatose Typ 1 (NF1) und anderen Krebsindikationen übertrifft.
- No treatment-related adverse events (TRAEs) or dose-limiting toxicities (DLTs) observed
- Long half-life of approximately 70 hours supports once-daily or less frequent oral dosing
- Potential for constant target inhibition while avoiding peak plasma toxicities
- Patient with stage 3 colon cancer achieved prolonged stable disease
- Linear pharmacokinetics observed with increased dose
- Favorable safety profile with no drug-related dose interruptions, reductions, or discontinuations
- None.
Insights
The Phase 1 clinical trial results for PAS-004 show promising initial data for this next-generation MEK inhibitor. Key findings include:
- Favorable safety profile with no treatment-related adverse events or dose-limiting toxicities observed at 2mg and 4mg doses
- Long half-life of ~70 hours, supporting once-daily or less frequent dosing
- Stable plasma concentrations at steady state, potentially allowing constant target inhibition while avoiding peak toxicities
- Preliminary efficacy signal with one heavily pre-treated colorectal cancer patient achieving prolonged stable disease
These results differentiate PAS-004 from first-generation MEK inhibitors, particularly in its potential for improved dosing regimens and reduced side effects like rash or GI toxicity. The linear pharmacokinetics and 70-hour half-life are significant improvements over existing NF1 treatments. While early, these data suggest PAS-004 could offer advantages in treating neurofibromatosis type 1, certain cancers and other MAPK-driven diseases. The initiation of the 8mg cohort and planned dosing schedule increase indicate confidence in the drug's tolerability.
The preliminary efficacy signal in a heavily pre-treated colorectal cancer patient with a BRAF K601E mutation is particularly noteworthy. This mutation lacks approved targeted therapies and colorectal cancer typically doesn't respond to single-agent MEK inhibitors. The patient's prolonged stable disease, extending into the 6th 28-day dosing cycle without toxicities, hints at PAS-004's potential broader applicability beyond NF1.
The absence of common MEK inhibitor side effects like rash, GI and ocular toxicities at the tested doses is encouraging. If maintained at higher doses, this could significantly improve patient quality of life and adherence compared to existing treatments. The pharmacokinetic profile, especially the consistent plasma levels at steady-state, suggests PAS-004 might achieve efficacious doses with a better safety profile than current options.
While these results are promising, it's important to see if the safety profile and efficacy signals hold up in larger patient cohorts and at higher doses. The upcoming data from the 8mg cohort will be critical in assessing PAS-004's full potential.
-- Single patient in 2mg cohort with stage 3 colon cancer who received 4 prior lines of therapy achieves prolonged stable disease and remains on drug into 6th dosing cycle --
-- No treatment-related adverse events (TRAEs) or dose-limiting toxicities (DLTs) observed to date, including no rash or gastrointestinal (GI) AEs --
-- Systemic exposure at steady-state enables constant target inhibition while avoiding peak plasma toxicities --
-- Half-life of approximately 70 hours supports once-daily or less frequent oral dosing --
-- Distinctive MEK inhibitor profile for the treatment of both NF1-related plexiform and cutaneous neurofibromas, cancer, and other opportunities --
MIAMI, Sept. 26, 2024 (GLOBE NEWSWIRE) -- Pasithea Therapeutics Corp. (NASDAQ: KTTA) (“Pasithea” or the “Company”), a clinical-stage biotechnology company developing PAS-004, a next-generation macrocyclic MEK inhibitor, for the treatment of neurofibromatosis type 1 (NF1) and other cancer indications, today announced safety, tolerability, pharmacokinetic (PK) and preliminary efficacy data from the first 2 cohorts of patients (n=6) in its Phase 1 clinical trial of PAS-004, being conducted at four clinical sites in the United States.
The Phase 1 clinical trial is a multi-center, open-label, dose escalation 3+3 study design to evaluate the safety, tolerability, pharmacokinetic (PK), pharmacodynamic (PD), and preliminary efficacy of PAS-004 in patients with MAPK pathway driven advanced solid tumors with a documented RAS, NF1 or RAF mutation or patients who have failed BRAF/MEK inhibition (NCT06299839).
“We are very pleased to share the PK, safety, and preliminary efficacy data from the 2 mg and 4 mg cohorts in our first-in-human Phase 1 clinical trial of PAS-004. We believe these data demonstrate a PK and safety profile that differentiates PAS-004 as a next-generation MEK inhibitor. We have already achieved significant PAS-004 exposures with a favorable safety profile and have not seen adverse side effects such as rash or GI toxicity, which are typical for MEK inhibitors even at low doses. The long half-life at approximately 70 hours, and the ability to achieve a flat PK curve at steady-state, aim to provide a constant target inhibition while avoiding peak plasma toxicities, which is a unique PK profile among MEK inhibitors used for the treatment of Neurofibromatosis type 1 (NF1),” stated Dr. Tiago Reis Marques, Chief Executive Officer of Pasithea.
“In addition, we are encouraged to see early potential signs of efficacy, with a heavily pre-treated patient with colorectal cancer showing prolonged stable disease. Colorectal cancer is known to not provide a RECIST response when treated with single-agent MEK inhibitors. This patient has a BRAF K601E mutation, a mutational status with no approved therapies. We are encouraged that this patient has been treated continuously into the 6th 28-day dosing cycle with no toxicities or AEs observed. While still early in clinical development, we believe PAS-004 is showing early signs of differentiation, indicating PAS-004 has the potential to outperform current MEK inhibitors in terms of safety, reduced administration frequency, and potentially efficacy. Our goal is to provide a once-daily or less frequent dosing treatment with broader application, not only for NF1 but also for other indications.”
Interim Phase 1 Results

Pharmacokinetics (PK)
- Plasma exposure increased with an increase in dose and linear PK is observed
- Long half-life of approximately 70 hours will allow for once daily dosing or longer intervals
- Prolonged systemic exposure with minimal fluctuation in PAS-004 plasma concentration at steady state (Cmax/Cmin ratio of 1.2) indicates a potential to achieve constant target inhibition
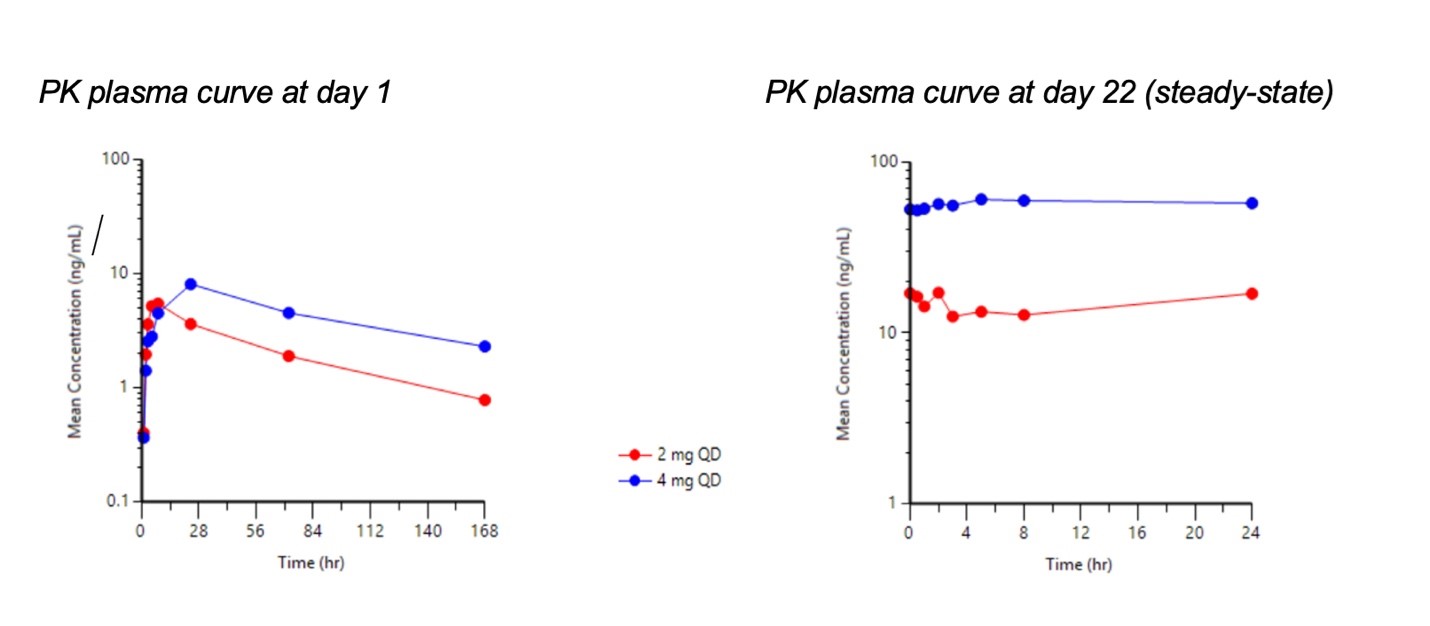
At steady-state, drug levels peaked at about 5 hours with a geometric mean maximum concentration (Cmax) of 16.2 and 61.3 ng/mL for the 2 mg and 4 mg dose groups, respectively. The mean elimination half-life was 67.9 hours supporting once-daily or less frequent oral dosing.
“PAS-004 has demonstrated distinct properties that we believe are significant advantages for an oral MEK inhibitor. PAS-004 has a significantly longer half-life compared to early-generation MEK inhibitors, particularly those used for the treatment of NF1, which have half-lives of less than 8 hours. The ability to achieve prolonged plasma exposures, as reflected in stable plasma concentrations at steady state, may potentially allow PAS-004 to achieve efficacious doses with a favorable safety profile,” stated Dr. Tiago Reis Marques, Chief Executive Officer of Pasithea.
Safety & Tolerability
- No treatment-related adverse events (TRAEs) or dose limiting toxicities (DLTs) observed to date
In the first 2 dosing cohorts (n=6), PAS-004 was shown to be well-tolerated with a favorable safety profile with no drug-related dose interruptions, reductions or discontinuations. There were no drug-related serious AEs (SAE) in any dose arm and no protocol-defined stopping criteria were met. Importantly, at the 2 and 4 mg dose levels no rash or skin toxicity, gastro-intestinal (GI) toxicity, or ocular toxicity have been observed to date.
The study independent Safety Review Committee has completed its safety review of data from the second dose cohort of 4 mg and the Company has initiated cohort 3 dosing at an increased dose of 8 mg in capsules and has filed a protocol amendment to increase dosing schedule.
PAS-004 Demonstrates a Differentiated MEK Inhibitor Profile
Unlike first-generation MEK inhibitors for the treatment of NF1 that require twice-daily dosing (BID) and exhibit short half-lives (<8 hours), PAS-004 has the potential to achieve prolonged target inhibition due to its long half-life of approximately 70 hours with once-daily dosing (QD). The PK profile shows consistent plasma levels at steady-state, as reflected by a low Cmax to Cmin ratio, potentially reducing the risks for Cmax-related toxicity. These findings provide a compelling rationale for the advancement of PAS-004 into clinical trials for both the treatment of cutaneous and plexiform neurofibromas in NF1, cancer and other MAPK-driven opportunities. The company expects to provide additional trial updates on a periodic basis as the trial progresses.
About Pasithea Therapeutics Corp.
Pasithea is a biotechnology company focused on the discovery, research and development of innovative treatments for central nervous system (CNS) disorders and RASopathies. With an experienced team of experts in the fields of neuroscience, translational medicine, and drug development, Pasithea is developing new molecular entities for the treatment of neurological disorders, including Neurofibromatosis type 1 (NF1), Solid Tumors, and Amyotrophic Lateral Sclerosis (ALS).
Forward Looking Statements
This press release contains statements that constitute “forward-looking statements” made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include statements regarding the Company’s ongoing Phase 1 clinical trial and the safety, tolerability, pharmacokinetic (PK) and preliminary efficacy of PAS-004, as well as all other statements, other than statements of historical fact, regarding the Company’s current views and assumptions with respect to future events regarding its business, as well as other statements with respect to the Company’s plans, assumptions, expectations, beliefs and objectives, the success of the Company’s current and future business strategies, product development, preclinical studies clinical studies, clinical and regulatory timelines, market opportunity, competitive position, business strategies, potential growth opportunities and other statements that are predictive in nature. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of the Company. While the Company believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to the Company on the date of this release. These forward-looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties, including risks that future clinical trial results may not match results observed to date, may be negative or ambiguous, or may not reach the level of statistical significance required for regulatory approval, as well as other factors set forth in the Company’s most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other filings made with the U.S. Securities and Exchange Commission (SEC). Thus, actual results could be materially different. The Company undertakes no obligation to update these statements whether as a result of new information, future events or otherwise, after the date of this release, except as required by law.
Pasithea Therapeutics Contact
Patrick Gaynes
Corporate Communications
pgaynes@pasithea.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/4765238d-9961-438e-b459-509a5c917974
https://www.globenewswire.com/NewsRoom/AttachmentNg/0e484fc9-7068-4c8f-8060-f4109228d013








