Incannex Announces Positive Topline Results from Phase 2 Psi-GAD1 Clinical Trial of Psilocybin in Generalised Anxiety Disorder
Highlights:
- PsiGAD1 trial achieves primary endpoint; PsiGAD psilocybin-assisted therapy demonstrated a statistically significant HAM-A reduction of 12.8 points from baseline, representing a 9.2-point improvement over psychotherapy with placebo (p <0.0001), exceeding the company’s expectations.
44% of patients in the psilocybin group demonstrated at least50% reduction in anxiety score and27% of patients showed disease remission– a remission rate more than 5 times higher than that of therapy with placebo.- Newly developed and formulated PSX-001 psilocybin drug product has been finalised - cGMP manufacture for clinical trial supply underway.
- Incannex to submit an Investigational New Drug (IND) application with U.S. Food and Drug Administration (FDA) to proceed to a multi-site Phase 2B trial.
MELBOURNE, Australia and NEW YORK, Feb. 28, 2024 (GLOBE NEWSWIRE) -- Incannex Healthcare Inc. (Nasdaq: IXHL), (Incannex or the Company), a leading cannabinoid and psychedelic medicine biotechnology company, is pleased to announce positive topline results from its Phase 2 Psi-GAD1 clinical trial of psilocybin in generalised anxiety disorder (GAD). The trial met its primary endpoint, demonstrating a large clinical effect in the psilocybin treatment group over the placebo group.
The trial protocol and treatment design were developed in partnership with the Clinical Psychedelic Lab at Monash University, led by Dr Paul Liknaitzky.
The reduction in HAM-A score from baseline in the psilocybin group was 12.8 points, from 29.5 at baseline to 16.8 at week 11 (6 weeks following the final dosing session), representing a decrease of 9.2 points over the placebo group (-12.8 psilocybin vs. -3.6 placebo; p<0.0001).
Psilocybin within the context of PsiGAD psychotherapy was observed to be well-tolerated, with only mild and moderate adverse events (AEs) reported. The reported AEs were consistent with the known effects of the drug. No serious or severe adverse events were observed. Only one person of the 73 participants withdrew from the trial during the 7-week treatment program.
“We are thrilled with the results from our initial PsiGAD trial,” said President and CEO Joel Latham. “This is the first time psilocybin has been investigated for treatment of generalised anxiety disorder, and the reduction in HAM-A scores we have observed are far greater those reported from trials on established medicines for treatment of anxiety. The improvement in anxiety scores in PsiGAD1 are of a similar magnitude to the change seen in studies investigating psilocybin for treatment of depression disorders. Safety is a key component of any new therapy, and we are delighted that no serious or severe adverse events were observed in PsiGAD patients, which is testament to the focus on safety within the PsiGAD treatment protocol. The safety and efficacy results from PsiGAD1 implore us to continue the development of PsiGAD through large scale well-controlled trials, because this treatment method has the potential to improve the quality of life for millions of people suffering from generalised anxiety disorder.”
Incannex have designed the follow-up Phase 2B clinical trial, PsiGAD2, with the assistance of Clerkenwell Health, a UK based contract research organisation specialising in psychiatry and CNS treatments. This trial will be conducted at multiple sites in the United States (US) and United Kingdom (UK).
In parallel, Incannex has finalised the development of formulation of its psilocybin drug product, PSX-001. Final preparations for the manufacture of the cGMP clinical trial supply of PSX-001 are underway. Documentation on the formulation development and cGMP manufacture will form the final pieces of the FDA IND application that Incannex commenced in August of 2023. Clearance of the IND by the agency is required for the Company to conduct the PsiGAD2 study at sites in the US.
Incannex is continuing to work with Clerkenwell Health to select trial sites and prepare the relevant regulatory documents for submission to the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK.
About Generalised Anxiety Disorder
Generalised Anxiety Disorder (GAD) is characterised by excessive anxiety and worry that occurs more days than not for at least 6 months and is not restricted to any particular environmental circumstances. Symptoms are variable, including feelings of persistent and excessive worry, nervousness, restlessness, difficulty concentrating, and a range of somatic manifestations. People with GAD find it difficult to control their worry, which may cause significant distress and impairment in social, occupational, or other areas of functioning. GAD is a relatively common disorder (about 6
This announcement has been approved for release to NASDAQ by the Incannex Board of Directors.
About Incannex Healthcare Inc.
Incannex is a clinical stage pharmaceutical development company that is developing unique medicinal cannabis pharmaceutical products and psychedelic medicine therapies for the treatment of obstructive sleep apnoea (OSA), traumatic brain injury (TBI) and concussion, lung inflammation (ARDS, COPD, asthma, bronchitis), rheumatoid arthritis, inflammatory bowel disease, anxiety disorders, addiction disorders, and pain, among other indications.
U.S. FDA approval and registration, subject to ongoing clinical success, is being pursued for each drug and therapy under development. Each indication under investigation currently has no, or limited, existing registered pharmacotherapy (drug) treatments available to the public and represent major global economic opportunities to Incannex and its shareholders.
Incannex has a strong patent filing strategy in place as it develops its products and therapies in conjunction with its medical and scientific advisory board and partners. The Company holds 19 granted patents and 30 pending patent applications. Incannex is listed on the NASDAQ as IXHL
Website: www.incannex.com.au
Investors: investors@incannex.com.au
Forward-looking statements
This press release contains "forward-looking statements" within the meaning of the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements are made as of the date they were first issued and were based on current expectations and estimates, as well as the beliefs and assumptions of management. The forward-looking statements included in this press release represent Incannex's views as of the date of this press release. Incannex anticipates that subsequent events and developments may cause its views to change. Incannex undertakes no intention or obligation to update or revise any forward-looking statements, whether as of a result of new information, future events or otherwise. These forward-looking statements should not be relied upon as representing Incannex's views as of any date after the date of this press release.
Contact Information:
Incannex Healthcare Inc.
Mr Joel Latham
Chief Executive Officer, President and Director
admin@incannex.com.au
Investor Relations Contact – United States
Laine Yonker
Edison Group
+1 (610) 716 2868
lyonker@edisongroup.com
Appendix: Comparison of PsiGAD Treatments to Existing Registered Treatments for Anxiety
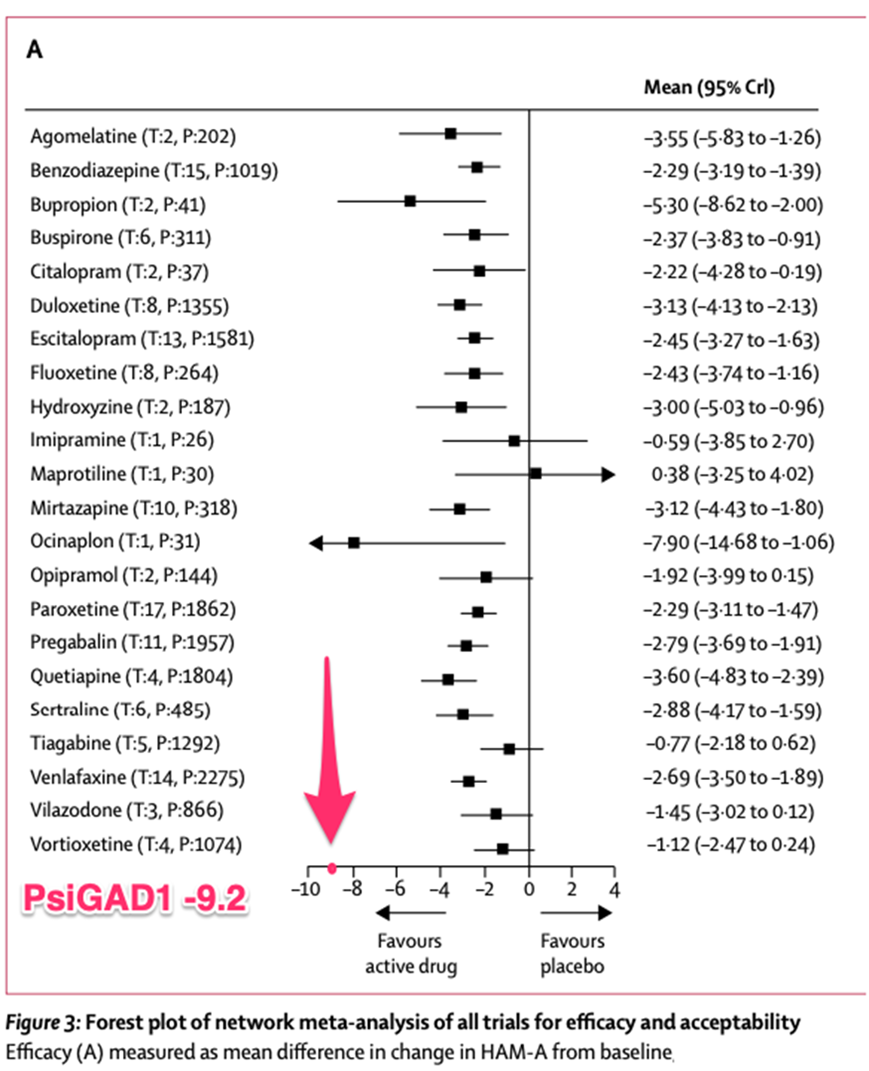
Figure: meta-analysis1 of psychotropic medications for GAD, as measured by the HAM-A; the best medication that was coded as having reliable (larger sample size) results in this analysis, quetiapine, has a between group difference in effect of -3.60 on the HAM-A. Note, some studies included in this meta-analysis were considered unreliable by the authors.
1 Slee, A., Nazareth, I., Bondaronek, P., Liu, Y., Cheng, Z., & Freemantle, N. (2019). Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. The Lancet, 393(10173), 768-777.
An infographic accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/bacb6159-feb6-4794-ae7f-2a0303debb18








