Innovent Presents at the 43rd Annual J.P. Morgan Healthcare Conference
Innovent Biologics presented at the 43rd Annual J.P. Morgan Healthcare Conference, highlighting 2025 as a pivotal year for business growth and global innovation. The company aims to achieve domestic product revenue of 20 billion RMB by 2027 through its expanding oncology portfolio and general biomedicine segment.
The company's innovation engine, Innovent Academy, has developed advanced technology platforms including ScFv engineering, T cell engager, and antibody platforms. They have progressed 8 ADC candidates into clinical trials with data from over 600 patients.
Key 2025 milestones include expected approvals for mazdutide for weight loss and type 2 diabetes, teprotumumab for thyroid eye disease, and picankibart for psoriasis. The company plans to submit NDAs or conduct registrational trials for seven drugs and initiate pivotal trials for seven innovative pipeline candidates.
Innovent Biologics si è presentata alla 43a Conferenza annuale J.P. Morgan Healthcare, sottolineando il 2025 come un anno cruciale per la crescita aziendale e l'innovazione globale. L'azienda mira a raggiungere un fatturato di prodotti domestici di 20 miliardi di RMB entro il 2027 attraverso il suo crescente portafoglio in oncologia e il segmento della biomedicina generale.
Il motore dell'innovazione dell'azienda, Innovent Academy, ha sviluppato piattaforme tecnologiche avanzate tra cui ingegneria ScFv, coinvolgimento delle cellule T e piattaforme di anticorpi. Hanno fatto avanzare 8 candidati ADC in trial clinici con dati provenienti da oltre 600 pazienti.
I principali traguardi del 2025 includono le approvazioni previste per mazdutide per la perdita di peso e il diabete di tipo 2, teprotumumab per la malattia oculare tiroidea, e picankibart per la psoriasi. L'azienda prevede di presentare NDA o condurre studi registrativi per sette farmaci e avviare trial pivotal per sette candidati innovativi in fase di sviluppo.
Innovent Biologics se presentó en la 43ª Conferencia Anual de Atención Médica de J.P. Morgan, destacando el 2025 como un año clave para el crecimiento empresarial y la innovación global. La compañía tiene como objetivo alcanzar ingresos por productos nacionales de 20 mil millones de RMB para 2027 a través de su creciente cartera de oncología y el segmento de biomedicina general.
El motor de innovación de la empresa, Innovent Academy, ha desarrollado plataformas tecnológicas avanzadas que incluyen ingeniería de ScFv, activadores de células T y plataformas de anticuerpos. Han avanzado 8 candidatos de ADC a ensayos clínicos con datos de más de 600 pacientes.
Los hitos clave de 2025 incluyen las aprobaciones esperadas para mazdutide para la pérdida de peso y la diabetes tipo 2, teprotumumab para la enfermedad ocular tiroidea, y picankibart para la psoriasis. La compañía planea presentar NDA o llevar a cabo ensayos de registro para siete medicamentos e iniciar ensayos importantes para siete candidatos innovadores en su cartera.
Innovent Biologics는 제43회 JP 모건 헬스케어 컨퍼런스에서 2025년이 비즈니스 성장과 글로벌 혁신의 중추적 해가 될 것이라고 강조했습니다. 이 회사는 2027년까지 20억 RMB의 국내 제품 수익 달성을 목표로 하고 있으며, 이는 확장되는 종양학 포트폴리오 및 일반 생의학 부문을 통해 이루어질 것입니다.
회사의 혁신 엔진인 Innovent Academy는 ScFv 엔지니어링, T 세포 결합 및 항체 플랫폼을 포함한 첨단 기술 플랫폼을 개발했습니다. 그들은 600명 이상의 환자 데이터를 바탕으로 8개의 ADC 후보를 임상 시험으로 진입시켰습니다.
2025년의 주요 이정표는 mazdutide의 체중 감소 및 제2형 당뇨병에 대한 승인, teprotumumab의 갑상선 안질환에 대한 승인, 그리고 picankibart의 건선 치료를 포함할 것으로 예상됩니다. 회사는 7개의 의약품에 대한 NDA를 제출하거나 등록 시험을 실시하고 7개의 혁신적인 파이프라인 후보에 대한 중요한 시험을 시작할 계획입니다.
Innovent Biologics s'est présenté à la 43ème Conférence Annuelle de la Santé de J.P. Morgan, mettant en avant l'année 2025 comme une année clé pour la croissance des affaires et l'innovation mondiale. L'entreprise vise à atteindre un revenu national de 20 milliards de RMB d'ici 2027 grâce à son portefeuille en oncologie et à son segment de biomédecine général en expansion.
Le moteur d'innovation de l'entreprise, Innovent Academy, a développé des plateformes technologiques avancées, notamment l'ingénierie ScFv, les engageurs de cellules T et les plateformes d'anticorps. Ils ont fait progresser 8 candidats ADC dans des essais cliniques avec des données provenant de plus de 600 patients.
Les jalons clés de 2025 incluent des approbations attendues pour mazdutide pour la perte de poids et le diabète de type 2, teprotumumab pour la maladie oculaire thyroïdienne, et picankibart pour le psoriasis. L'entreprise prévoit de soumettre des NDA ou de mener des études d'enregistrement pour sept médicaments et de commencer des essais décisifs pour sept candidats innovants dans son pipeline.
Innovent Biologics stellte auf der 43. J.P. Morgan Healthcare Conference vor und hob 2025 als ein entscheidendes Jahr für das Unternehmenswachstum und globale Innovation hervor. Das Unternehmen strebt an, bis 2027 einen Umsatz von 20 Milliarden RMB aus inländischen Produkten zu erreichen, durch sein wachsendes Onkologieportfolio und den allgemeinen Biomedizin-Sektor.
Der Innovationsmotor des Unternehmens, Innovent Academy, hat fortschrittliche Technologieplattformen entwickelt, darunter ScFv-Engineering, T-Zell-Verknüpfung und Antikörperplattformen. Sie haben 8 ADC-Kandidaten in klinische Studien überführt, mit Daten von über 600 Patienten.
Wichtige Meilensteine im Jahr 2025 umfassen voraussichtliche Genehmigungen für mazdutide zur Gewichtsreduktion und für Typ-2-Diabetes, teprotumumab bei der Thyroid-Augenkrankheit sowie picankibart bei Psoriasis. Das Unternehmen plant, NDAs einzureichen oder registrierende Studien für sieben Arzneimittel durchzuführen und entscheidende Studien für sieben innovative Pipeline-Kandidaten zu starten.
- Targeting 20 billion RMB domestic product revenue by 2027
- 8 ADC candidates in clinical trials with data from 600+ patients
- Multiple products expected for approval in 2025: mazdutide, teprotumumab, and picankibart
- Picankibart achieved over 80% PASI 90 within 16 weeks in clinical trials
- Plans for 14 drugs in various stages of registrational trials or NDAs in 2025
- None.
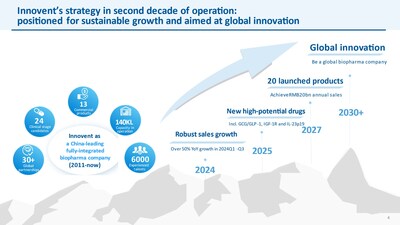
Advancing into new stage of sustainable growth and global innovation
During the conference, Dr. De-Chao Michael Yu, Founder, Chairman and CEO of Innovent, delivered a presentation highlighting 2025 as a pivotal year for significantly business growth and concreate steps in global innovation. These efforts will further strengthen the foundation for Innovent's vision of becoming a global premier biopharma company.
Clear path to sustainable growth
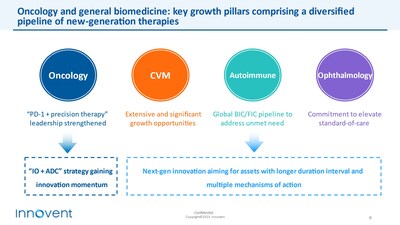
Innovent has established itself as a leading brand in oncology, consistently gaining momentum with an expanding portfolio of synergistic products. Additionally, another key growth driver, the general biomedicine segment, features a highly competitive product lineup, poised to unlock the substantial opportunities in chronic disease areas.
The company remains confident in achieving its domestic product revenue target of
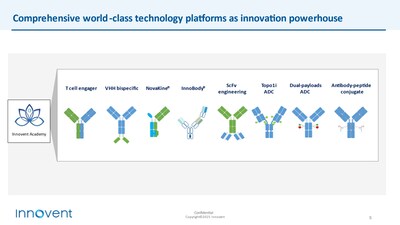
Focus on world-class technology platforms and key therapeutic areas
Innovent Academy, the company's innovation engine, has built a world-class technology platform, encompassing ScFv engineering, T cell engager (TCE), VHH bispecific antibodies, Topo1i ADC, dual payload ADC, and antibody peptide conjugates (APC). These platforms have consistently delivered innovative molecules, providing a driving force for the company's long-term development.
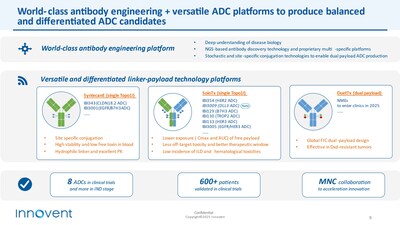
In particular, Innovent combines its world-class antibody engineering and multiple sets of differentiated linker payload technologies to create the TOPO1i ADC technology platform (SoloTx) and dual payload ADC technology platform (DuetTx), producing a pipeline of potential best-in-class (BIC) or first-in-class (FIC) ADC candidates.
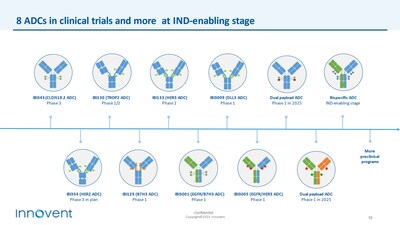
The company has so far advanced 8 ADC candidates into clinic trials, supported by efficacy and safety data from over 600 patients, with multiple ADCs receiving breakthrough therapy designation (BTD). This further validates the differentiated advantage of the company's ADC platform technology and its strong clinical execution.
Innovent continues to focus on high-potential therapeutic areas in oncology and general biomedicine. The "PD-1+precision therapies" pipeline strengthens its leadership in oncology, while the "IO+ADC" strategy is set to transform cancer treatment. Meanwhile, its general biomedicine pipeline covers next generation treatments for autoimmune, cardiovascular and metabolic, and ophthalmic diseases, aiming to elevate treatment standards for diverse patient populations.
Embracing new opportunities in global innovation
Innovent Academy's advancements have paved the way for a globally competitive pipeline of next generation IO and ADC candidates. The company plans to expand into innovative ADCs, bispecific (multi-specific) antibodies, and next-generation autoimmune and CVM therapies for global development.
- IBI343 (CLDN18.2 ADC): Phase 3 multi-regional clinical trial (MRCT) has been initiated in
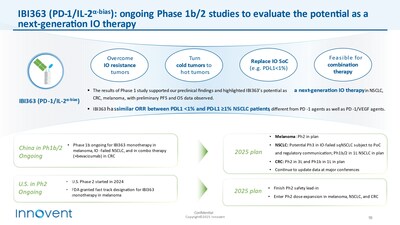
China andJapan for gastric cancer. Pancreatic cancer MRCT clinical Phase 1 data shows positive efficacy and safety signals in Chinese patients and has started patient enrollment in theU.S. Pivotal clinical trials are anticipated in 2025, subject to PoC validation. - IBI363:This first-in-class PD-1/IL-2α-biased bispecific antibody has shown promising Phase 1 clinical data, obtained from hundreds of patients in IO resistant non-small cell lung cancer (NSCLC), melanoma, and IO unresponsive "cold tumor" colorectal cancer (CRC). This points to its potential as the next generation IO cornerstone drug. Innovent is following up on the Phase 1b/2 expansion cohorts of these cancer types. Pivotal clinical studies in IO-resistant advanced squamous NSCLC and IO-naïve advanced melanoma are planned to launch in
China in 2025, subject to PoC data and regulatory communication. A Phase 2 clinical study is ongoing in theU.S. and will further expand into cohorts for NSCLC, CRC, and melanoma. - IBI3009 (DLL3 ADC): Through global licensing collaboration with Roche, Innovent aims to accelerate the development of this potentially best-in-class DLL3 ADC for small cell lung cancer patients worldwide.
2025 outlook: unlocking growth opportunities
Looking ahead in 2025, the company anticipates a year of rapid growth, driven by six new drug launches and further advancements in commercialization across oncology and general biomedicine. Key highlights include:
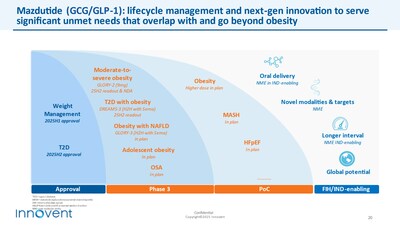
- Mazdutide (GCG/GLP-1): Expected approvals for weight loss and type 2 diabetes indications in the first and second half of 2025, respectively, will provide a best-in-class GCG/GLP-1 dual agonist drug offering robust weight loss and glucose reduction, substantial liver fat reduction as well as comprehensive metabolic benefits for the vast obese, overweight and diabetes population;
- Teprotumumab (IGF-1R): Anticipated launch as
China's first anti-IGF-1R monoclonal antibody for thyroid eye disease (TED), addressing a 60-year treatment gap. - Picankibart (IL-23p19): Approval anticipated by late 2025. Picankibart is the first globally to report that over
80% of subjects achieved PASI 90 (≥90% improvement in psoriasis area and severity index) within 16 weeks of treatment. It also demonstrates clear advantages, including sustained long-term efficacy, effective in patients resistant to IL-17 inhibitors, and flexibility with seasonal dosing intervals. Picankibart is poised to deliver exceptional comprehensive benefits to psoriasis patients inChina .
As the company advances key cornerstone products in its pipeline, we are actively expanding the development of mazdutide, teprotumumab, and picankibart into additional indications to maximize the portfolio's value. Building on this solid foundation, next-generation candidates are gradually entering clinical development, aiming to address global challenges related to aging and chronic disease burden. These innovations focus on extending dosing intervals, oral delivery, and novel mechanisms of action.
Looking ahead to 2025, the company plans to submit NDAs or conduct ongoing registrational trials for seven drugs, while initiating pivotal or registrational trials for seven innovative pipeline candidates, pending PoC results. Additionally, molecules with global potential and novel mechanisms of action (MoAs) will progress into PoC and first-in-human studies. These efforts solidify Innovent's vision of "growing into a global premier biopharma company."
Scan the QR code for presentation slides
About Innovent
Innovent is a leading biopharmaceutical company founded in 2011 with the mission to empower patients worldwide with affordable, high-quality biopharmaceuticals. The company discovers, develops, manufactures and commercializes innovative medicines that target some of the most intractable diseases. Its pioneering therapies treat cancer, cardiovascular and metabolic, autoimmune and eye diseases. Innovent has launched 13 products in the market. It has 4 new drug applications under regulatory review, 3 assets in Phase III or pivotal clinical trials and 17 more molecules in early clinical stage. Innovent partners with over 30 global healthcare companies, including Eli Lilly, Sanofi, Incyte, Adimab, LG Chem and MD Anderson Cancer Center.
Guided by the motto, "Start with Integrity, Succeed through Action," Innovent maintains the highest standard of industry practices and works collaboratively to advance the biopharmaceutical industry so that first-rate pharmaceutical drugs can become widely accessible. For more information, visit www.innoventbio.com, or follow Innovent on Facebook and LinkedIn.
Forward-Looking Statements
This news release may contain certain forward-looking statements that are, by their nature, subject to significant risks and uncertainties. The words "anticipate", "believe", "estimate", "expect", "intend" and similar expressions, as they relate to Innovent, are intended to identify certain of such forward-looking statements. Innovent does not intend to update these forward-looking statements regularly.
These forward-looking statements are based on the existing beliefs, assumptions, expectations, estimates, projections and understandings of the management of Innovent with respect to future events at the time these statements are made. These statements are not a guarantee of future developments and are subject to risks, uncertainties and other factors, some of which are beyond Innovent's control and are difficult to predict. Consequently, actual results may differ materially from information contained in the forward-looking statements as a result of future changes or developments in our business, Innovent's competitive environment and political, economic, legal and social conditions.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/innovent-presents-at-the-43rd-annual-jp-morgan-healthcare-conference-302353728.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/innovent-presents-at-the-43rd-annual-jp-morgan-healthcare-conference-302353728.html
SOURCE Innovent Biologics