Innovent Announces Mazdutide Demonstrates 80.2% Reduction in Liver Fat Content in Exploratory Analysis of Phase 3 Weight Management GLORY-1 Study at ADA 2024
Innovent Biologics announced results from their Phase 3 GLORY-1 study, revealing a significant reduction in liver fat content using mazdutide, a GLP-1R and GCGR dual agonist. The study involved 610 Chinese adults with obesity or overweight, showing an average liver fat reduction of 80.2% in participants with a baseline LFC ≥10%. The 48-week trial demonstrated dose-dependent improvements in liver fat content, body weight, and other metabolic parameters, outperforming placebo. These findings suggest mazdutide's potential in treating MAFLD and MASH.
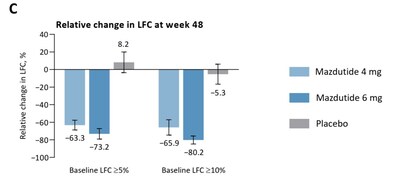
- Mazdutide demonstrated an 80.2% reduction in liver fat content for participants with a baseline LFC ≥10%.
- Significant dose-dependent liver fat content reduction: 63.3% for 4 mg and 73.2% for 6 mg doses compared to 8.2% for placebo.
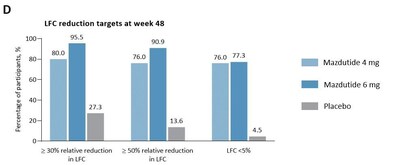
- High effectiveness: 95.5% of participants on 6 mg mazdutide achieved ≥30% liver fat reduction, with 77.3% reaching LFC <5%.
- Marked improvements in body weight, waist circumference, blood pressure, transaminase, and lipid levels.
- None.
SAN FRANCISCO and
Mazdutide (Innovent R&D code: IBI362) is a glucagon-like peptide-1 receptor (GLP-1R) and glucagon receptor (GCGR) dual agonist. By activating GLP-1R, it reduces appetite and delays gastric emptying, thereby achieving weight loss. At the same time, through activation of GCGR, it increases energy expenditure, enhances fatty acid oxidation and lipolysis, and reduces liver fat.
Professor Linong Ji, the leading principal investigator of the study, Peking University People's Hospital, stated, "Metabolic dysfunction-associated fatty liver disease (MAFLD) is one of the most common chronic liver diseases in the world and the most common comorbidity in overweight and obese people in
In GLORY-1 trial (ClinicalTrial.gov:NCT05607680), 610 Chinese adults with BMI ≥28 kg/m2, or ≥24 kg/m2 and at least one weight-related comorbidity were randomized in a 1:1:1 ratio to receive once-weekly, subcutaneous mazdutide 4 mg, 6 mg or placebo for 48 weeks.
A total of 92 participants had an MRI scan and a liver fat content (LFC) measurement by MRI-PDFF at baseline, among whom 69 participants (25 with mazdutide 4 mg, 22 with mazdutide 6 mg and 22 with placebo) had LFC ≥
Mazdutide treatment led to robust reduction in LFC and improved liver function
- In 69 participants with baseline LFC ≥
5% and week 48 LFC assessment, treatment with mazdutide for 48 weeks dose-dependently reduced LFC, substantially greater than placebo (mean relative change from baseline: −63.3% for mazdutide 4 mg; −73.2% for mazdutide 6 mg;8.2% for placebo) - In participants with baseline LFC ≥
10% , treatment with mazdutide 6 mg led to mean relative reduction of80.2% in LFC at week 48.
- Compared with placebo, substantially more participants with mazdutide achieved ≥
30% relative reduction in LFC, ≥50% relative reduction in LFC and normalization of LFC (<5% ) at week 48. Of note,95.5% of participants with mazdutide 6 mg achieved ≥30% relative reduction in LFC at week 48, and77.3% of participants with mazdutide 6mg achieved LFC<5% at week 48.
- Marked reductions in body weight, waist circumference, blood pressure, transaminase and lipids were observed in participants treated with mazdutide.
Dr. Lei Qian, Vice President of Clinical Development of Innovent, stated, "Improvement of liver fat metabolism is one of the important advantages of mazdutide as dual against of GLP-1R/GCGR. Consistent with observed positive signs of liver fat reduction in the Phase 2 study of mazdutide, the results of the GLORY-1 study further support that mazdutide may promote liver fat metabolism through the stimulation of GCGR and significantly reduce liver fat content. We believe mazdutide's exceptional performance in reducing intrahepatic fat shows its promising potential, warranting further exploration of the drug's clinical value in MAFLD and MASH diseases. We hope to provide improved treatment options for the large population affected by metabolic-related fatty liver disease."
About Obesity
As a chronic disease with a complex etiology, obesity is a leading risk factor of type 2 diabetes, fatty liver, cardiovascular and cerebrovascular diseases, kidney diseases, joint diseases, sleep apnea and various cancers. With economic development and lifestyle changes, the number of obese populations in
About Mazdutide (IBI362)
Innovent entered into an exclusive license agreement with Eli Lilly and Company (Lilly) for the development and potential commercialization of OXM3 (also known as mazdutide), a GLP-1R and GCGR dual agonist, in
In February 2024, the first NDA of mazdutide was accepted by the CDE of
About Innovent
Innovent is a leading biopharmaceutical company founded in 2011 with the mission to empower patients worldwide with affordable, high-quality biopharmaceuticals. The company discovers, develops, manufactures and commercializes innovative medicines that target some of the most intractable diseases. Its pioneering therapies treat cancer, cardiovascular and metabolic, autoimmune and eye diseases. Innovent has launched 10 products in the market. It has 4 new drug applications under regulatory review, 4 assets in Phase III or pivotal clinical trials and 18 more molecules in early clinical stage. Innovent partners with over 30 global healthcare companies, including Eli Lilly, Sanofi, Incyte, Adimab, LG Chem and MD Anderson Cancer Center.
Guided by the motto, "Start with Integrity, Succeed through Action," Innovent maintains the highest standard of industry practices and works collaboratively to advance the biopharmaceutical industry so that first-rate pharmaceutical drugs can become widely accessible. For more information, visit www.innoventbio.com, or follow Innovent on Facebook and LinkedIn.
Forward-looking statement
This news release may contain certain forward-looking statements that are, by their nature, participant to significant risks and uncertainties. The words "anticipate", "believe", "estimate", "expect", "intend" and similar expressions, as they relate to Innovent Biologics ("Innovent"), are intended to identify certain of such forward-looking statements. The Company does not intend to update these forward-looking statements regularly.
These forward-looking statements are based on the existing beliefs, assumptions, expectations, estimates, projections and understandings of the management of the Company with respect to future events at the time these statements are made. These statements are not a guarantee of future developments and are participant to risks, uncertainties and other factors, some of which are beyond the Company's control and are difficult to predict. Consequently, actual results may differ materially from information contained in the forward-looking statements as a result of future changes or developments in our business, the Company's competitive environment and political, economic, legal and social conditions.
The Company, the Directors and the employees of the Company assume (a) no obligation to correct or update the forward-looking statements contained in this site; and (b) no liability in the event that any of the forward-looking statements does not materialise or turn out to be incorrect.
References
[i] Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in |
[ii] Institute for Health Metrics and Evaluation. Global Health Data Exchange. GBD results tool. http://ghdx.healthdata.org/gbd-resultstool (accessed Jan 10, 2021). |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/innovent-announces-mazdutide-demonstrates-80-2-reduction-in-liver-fat-content-in-exploratory-analysis-of-phase-3-weight-management-glory-1-study-at-ada-2024--302180984.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/innovent-announces-mazdutide-demonstrates-80-2-reduction-in-liver-fat-content-in-exploratory-analysis-of-phase-3-weight-management-glory-1-study-at-ada-2024--302180984.html
SOURCE Innovent Biologics