Iveric Bio Receives FDA Agreement Under Special Protocol Assessment (SPA) for GATHER2 Phase 3 Clinical Trial of Zimura® in Geographic Atrophy Secondary to Age-Related Macular Degeneration
IVERIC bio, Inc. (Nasdaq: ISEE) today announced that the Company received written agreement from the U.S. Food and Drug Administration (FDA) under a Special Protocol Assessment (SPA) for the overall design of GATHER2, the Company’s pivotal clinical trial of Zimura® (avacincaptad pegol) in development for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). As previously announced, the Company expects to complete enrollment in GATHER2 in late July of this year. Based on this timeline, the Company expects topline GATHER2 data to be available in the second half of 2022, approximately one year after the enrollment of the last patient plus the time needed for database lock and analysis.
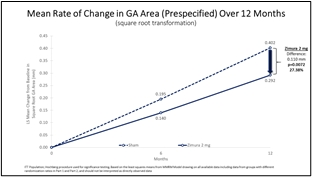
Zimura 2 mg vs. Sham: Mean Rate of Change in GA Area (Prespecified) Over 12 Months (Graphic: Business Wire)
Similar to the Company’s completed GATHER1 clinical trial, GATHER2 is designed to be an adequate and well-controlled clinical trial which, if positive, would support a New Drug Application (NDA) for Zimura in the treatment of GA secondary to AMD. The SPA agreement further solidifies the Company’s plans to file an application with the FDA if the ongoing GATHER2 clinical trial meets its primary efficacy endpoint at 12 months.
In connection with the SPA, the FDA recommended, and the Company accepted, modifying the primary efficacy endpoint for the GATHER2 trial from the mean rate of change in GA area over 12 months measured by fundus autofluorescence (FAF) at three timepoints: baseline, month 6 and month 12, to the mean rate of growth (slope) estimated based on GA area measured by FAF in at least three timepoints: baseline, month 6 and month 12. In connection with the SPA, the Company submitted, and the FDA reviewed, a revised clinical trial protocol and statistical analysis plan (SAP) for the GATHER2 trial reflecting the revised primary efficacy endpoint and agreed upon statistical analysis method. The original primary efficacy endpoint estimated the mean rate of change in GA area from baseline to month 12, as measured by FAF readings at three time points (baseline, month 6 and month 12), without assuming a constant rate of growth over the period. Using the same raw data, the FDA preferred method estimates the mean rate of growth of GA area from baseline to month 12, based on FAF readings at the same three time points (baseline, month 6 and month 12), assuming a constant rate of growth over the period, essentially fitting a straight line based on the data.
“We are excited to receive this SPA agreement from the FDA,” stated Pravin U. Dugel, MD, President of Iveric Bio. “We thank the FDA for their collaborative interactions and valuable input on the primary efficacy endpoint for the GATHER2 trial, which we believe reflects the FDA’s current thinking. The modification of the primary efficacy endpoint does not require collecting any new data but instead reflects a change in how the data are analyzed. We look forward to continuing to work with the FDA and following their guidance as we work toward preparing the eventual NDA submission for Zimura.”
In parallel discussions with those for the GATHER2 SPA, the FDA indicated to the Company that, as part of a future NDA submission for Zimura, the GATHER1 results will be considered using the original prespecified primary efficacy endpoint analysis, together with a post-hoc analysis using the same FDA preferred method that will be used for the GATHER2 trial (mean rate of growth (slope) estimated based on GA area measured by FAF in the relevant timepoints).
Dr. Dugel stated, “We are pleased that the FDA agrees the design and planned analysis of GATHER2 as modified adequately address the objectives necessary to support an NDA submission. We are also pleased that as part of a future NDA submission, the FDA has indicated that it will consider the GATHER1 data using the original prespecified primary efficacy endpoint analysis, together with the new FDA preferred method that we are using for GATHER2. The data from GATHER1, when analyzed on a post-hoc basis using the FDA’s preferred method, show results that are highly consistent with and strongly supportive of the results we originally reported for GATHER1. We continue to believe GATHER1 will serve as one of the two adequate and well-controlled pivotal clinical trials required for purposes of obtaining marketing approval of Zimura in GA.”
Below are the month 12 and month 18 results for both analyses for the GATHER1 trial:
Zimura 2 mg vs. Sham
Mean Rate of Change in GA Area (prespecified) & Mean Rate of GA Growth (Slope) (FDA)
The accompanying graphs illustrate the 12-month results for the Zimura 2 mg group as compared to the corresponding sham group, using both the original prespecified primary efficacy endpoint analysis and the new FDA preferred method that will be used for the GATHER2 trial.
Below are the month 12 and month 18 results for both analyses for the GATHER1 trial, for the Zimura 2 mg group as compared to its corresponding sham group:
MRM Analysis |
Zimura 2 mg (N = 67) |
Sham (N = 110) |
Difference |
% Difference |
P-Value |
12 Month Sq. Rt. Transformation: |
|
|
|
|
|
Mean Rate of Change in GA Area (mm) |
0.292 |
0.402 |
0.110 |
|
0.0072(a) |
Mean Rate of GA Growth (Slope) (mm) |
0.283 |
0.392 |
0.109 |
|
0.0063(b) |
12 Month Observed Data: |
|
|
|
|
|
Mean Rate of Change in GA Area (mm2) |
1.592 |
2.290 |
0.697 |
|
0.0059(b) |
Mean Rate of GA Growth (Slope) (mm2) |
1.221 |
1.889 |
0.668 |
|
0.0050(b) |
18 Month Sq. Rt. Transformation: |
|
|
|
|
|
Mean Rate of Change in GA Area (mm) |
0.430 |
0.599 |
0.168 |
|
0.0014(b) |
Mean Rate of GA Growth (Slope) (mm) |
0.451 |
0.607 |
0.156 |
|
0.0027(b) |
18 Month Observed Data: |
|
|
|
|
|
Mean Rate of Change in GA Area (mm2) |
2.431 |
3.587 |
1.156 |
|
0.0009(b) |
Mean Rate of GA Growth (Slope) (mm2) |
1.914 |
2.951 |
1.037 |
|
0.0023(b) |
The estimates for the Zimura 2 mg group vs. sham are from the MRM model, drawing on all available data, including data from groups with different randomization ratios in Part 1 and Part 2 of the trial, and should not be interpreted as directly observed data.
(a) Prespecified primary endpoint; statistically significant
(b) Descriptive p-value
Zimura 4 mg vs. Sham
Mean Rate of Change in GA Area (prespecified) & Mean Rate of GA Growth (Slope) (FDA)
The accompanying graphs illustrate the 12-month results for the Zimura 4 mg group as compared to the corresponding sham group, using both the original prespecified primary efficacy endpoint analysis and the new FDA preferred method that will be used for the GATHER2 trial.
Below are the month 12 and month 18 results for both analyses for the GATHER1 trial, for the Zimura 4 mg group as compared to its corresponding sham group:
MRM Analysis |
Zimura 4 mg (N = 83) |
Sham (N = 84) |
Difference |
% Difference |
P-Value |
12 Month Sq. Rt. Transformation: |
|
|
|
|
|
Mean Rate of Change in GA Area (mm) |
0.321 |
0.444 |
0.124 |
|
0.0051(a) |
Mean Rate of GA Growth (Slope) (mm) |
0.307 |
0.416 |
0.109 |
|
0.0100(b) |
12 Month Observed Data: |
|
|
|
|
|
Mean Rate of Change in GA Area (mm2) |
2.061 |
2.770 |
0.709 |
|
0.0082(b) |
Mean Rate of GA Growth (Slope) (mm2) |
1.674 |
2.273 |
0.599 |
|
0.0147(b) |
18 Month Sq. Rt. Transformation: |
|
|
|
|
|
Mean Rate of Change in GA Area (mm) |
0.391 |
0.559 |
0.167 |
|
0.0021(b) |
Mean Rate of GA Growth (Slope) (mm) |
0.373 |
0.512 |
0.139 |
|
0.0086(b) |
18 Month Observed Data: |
|
|
|
|
|
Mean Rate of Change in GA Area (mm2) |
2.460 |
3.486 |
1.026 |
|
0.0034(b) |
Mean Rate of GA Growth (Slope) (mm2) |
2.142 |
3.010 |
0.868 |
|
0.0106(b) |
(a) Prespecified primary endpoint; statistically significant
(b) Descriptive p-value
Safety results from GATHER1 were not impacted as part of this analysis. As previously reported, the most frequently reported ocular adverse events were related to the injection procedure. The investigator-reported incidence of choroidal neovascularization (CNV) in the sham group was 3 patients (
As previously announced, the Company is only advancing the Zimura 2 mg dose in the GATHER2 clinical trial.
The Company plans to make additional supportive information regarding the GATHER1 post-hoc analysis available in its public filings with the Securities and Exchange Commission.
“This written agreement from the FDA is consistent with our clinical and regulatory strategy and, we believe, reaffirms our planned approval pathway for Zimura for the treatment of GA secondary to AMD,” stated Glenn P. Sblendorio, Chief Executive Officer of Iveric Bio. “With patient enrollment and retention exceeding our expectations, we are poised to complete enrollment in the ongoing GATHER2 clinical trial in late July of this year. We believe we are on track for initial, topline data from GATHER2 to be available approximately one year after the enrollment of the last patient in the trial, plus the time needed for database closure and analysis.”
About Special Protocol Assessments
The SPA process is a procedure by which the FDA provides a clinical trial sponsor with an official evaluation and written guidance on the design of a proposed protocol intended to form the basis for a new drug application.
A SPA does not ensure the receipt of marketing approval or that the approval process will be faster than conventional regulatory procedures. Final marketing approval depends on efficacy and safety results and an evaluation of the overall benefits and risks of treatment after review of the data from the development program in its totality.
The SPA agreement may only be changed through a written agreement between the sponsor and the FDA, or if the FDA becomes aware of a substantial scientific issue essential to product efficacy or safety. For more information on Special Protocol Assessments, please visit: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/special-protocol-assessment-guidance-industry.
About Zimura
Zimura (avacincaptad pegol) is an investigational drug product and has not been approved for use anywhere globally. Zimura is designed to target and inhibit the cleavage of complement protein C5 and the formation of its downstream fragments, C5a and C5b. By inhibiting the formation of these fragments, Zimura is believed to decrease or slow the chronic inflammation and cell death associated with the retinal aging process by decreasing the formation of membrane attack complex (MAC) and inflammasome activity, thereby potentially avoiding or slowing the degeneration of retinal pigment epithelial cells. This potential mechanism is the rationale for Zimura as a potential therapy for geographic atrophy secondary to age-related macular degeneration.
Conference Call/Web Cast Information
IVERIC bio’s management team will host a conference call/webcast today at 8:00 a.m. Eastern Time to discuss today’s announcement. To participate in the conference call, dial 1-888-317-6003 (USA) or 1-412-317-6061 (International), passcode 9482414 . A live, listen-only audio webcast of the conference call can be accessed on the Investors section of the IVERIC bio website at www.ivericbio.com. A replay will be available approximately two hours following the live call for two weeks. The replay number is 1-877-344-7529 (USA Toll Free), passcode 10158299.
About IVERIC bio
IVERIC bio is a science-driven biopharmaceutical company focused on the discovery and development of novel treatment options for retinal diseases with significant unmet medical needs. The Company is currently developing both therapeutic product candidates for age-related retinal diseases and gene therapy product candidates for orphan inherited retinal diseases. For more information on the Company, please visit www.ivericbio.com.
Forward-looking Statements
Any statements in this press release about the Company’s future expectations, plans and prospects constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Forward-looking statements include any statements about the Company’s strategy, future operations and future expectations and plans and prospects for the Company, and any other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend”, “goal,” “future”, “may”, “might,” “plan,” “predict,” “project,” “seek,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions. In this press release, the Company’s forward looking statements include statements about the Company’s development and regulatory strategy for Zimura, including its strategy to submit an NDA to and seek marketing approval from the FDA for Zimura for the treatment of GA secondary to AMD if the ongoing GATHER2 clinical trial meets its primary efficacy endpoint at 12 months, the timing, progress and results of clinical trials, including expectations regarding patient enrollment and retention in GATHER2 and the availability of topline data from that trial, and other research and development activities and the potential utility of Zimura. Such forward-looking statements involve substantial risks and uncertainties that could cause the Company’s development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, those related to expectations for regulatory matters, the progression and duration of the COVID-19 pandemic and responsive measures thereto and related effects on the Company’s research and development programs, operations and financial position, the initiation and the progress of research and development programs and clinical trials, including enrollment and retention in clinical trials, availability of data from these programs, reliance on clinical trial sites, contract research organizations and other third parties, establishment of manufacturing capabilities, developments from the Company’s competitors and the marketplace for its products, need for additional financing and negotiation and consummation of business development transactions and other factors discussed in the “Risk Factors” section contained in the quarterly and annual reports that the Company files with the Securities and Exchange Commission. Any forward-looking statements represent the Company’s views only as of the date of this press release. The Company anticipates that subsequent events and developments may cause its views to change. While the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so except as required by law.
ISEE-G
View source version on businesswire.com: https://www.businesswire.com/news/home/20210706005200/en/







