Iveric Bio Announces Post-Hoc Analysis from GATHER1 Clinical Trial of Zimura® at American Society of Retina Specialists Meeting
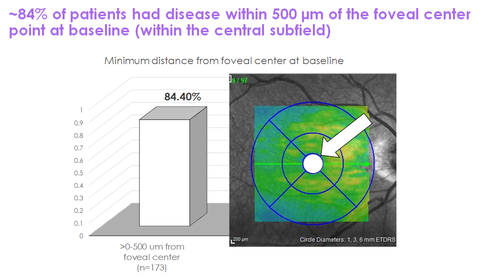
IVERIC bio, Inc. (ISEE) announced promising results from a post-hoc analysis of the GATHER1 clinical trial, demonstrating that Zimura significantly reduced geographic atrophy (GA) lesion growth compared to a sham treatment. This analysis highlighted that 84% of patients had lesions close to the foveal center, reinforcing Zimura's potential benefits for a broad patient population. Safety data showed no drug-related adverse events. The company is set to release topline data from the GATHER2 trial in Q3 2022, with plans to seek regulatory approval if results are favorable.
- Zimura showed significant reduction of GA lesion growth in post-hoc analysis of GATHER1 trial.
- 84% of patients had lesions within 500 microns of the foveal center, indicating broad applicability of Zimura.
- No drug-related adverse events were reported during the GATHER1 trial.
- None.
Insights
Analyzing...
(Graphic: Business Wire)
“The vast majority of patients in GATHER1 had GA lesions within the area that clinicians are most concerned about protecting,” said
“The multiple post-hoc analyses from GATHER1 continue to provide consistent results, which give us a great deal of confidence in the robustness of the GATHER1 data and the potential of Zimura as a treatment to help a broad patient population with GA,” stated
The analysis reported that approximately
The most frequently reported ocular adverse events were related to the injection procedure. There were no drug related adverse events such as inflammation or endophthalmitis reported in GATHER1. No additional safety analysis was performed as part of this post-hoc analysis.
The full set of slides of the presentation is available on the Company’s website at https://investors.ivericbio.com/events-and-presentation.
About GATHER1 and GATHER2
The Company previously announced that in GATHER1, Zimura (avacincaptad pegol) met its pre-specified primary efficacy endpoint with statistical significance. The most frequently reported ocular adverse events in this trial were related to the injection procedure. The Company expects topline data for GATHER2, a second Phase 3 clinical trial for Zimura for GA, to be available in the third quarter of 2022, approximately one year after the enrollment of the last patient in the trial plus the time needed for database lock and analysis. If 12-month results from GATHER2 are positive, the Company plans to submit applications with the
About Zimura
Zimura (avacincaptad pegol) is an investigational drug product and has not been approved for use anywhere globally. Zimura is designed to target and inhibit the cleavage of complement protein C5 and the formation of its downstream fragments, C5a and C5b. By inhibiting the formation of these fragments, Zimura is believed to decrease or slow the chronic inflammation and cell death associated with the retinal aging process by decreasing the formation of membrane attack complex (MAC) and inflammasome activity, thereby potentially avoiding or slowing the degeneration of retinal pigment epithelial cells. This potential mechanism is the rationale for Zimura as a potential therapy for geographic atrophy.
About
Forward-looking Statements
Any statements in this press release about Iveric Bio’s future expectations, plans and prospects constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Forward-looking statements include any statements about the Company’s strategy, future operations and future expectations and plans and prospects for the Company, and any other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend”, “goal,” “may”, “might,” “plan,” “predict,” “project,” “seek,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions. In this press release, the Company’s forward looking statements include statements about its expectations regarding its development and regulatory strategy for Zimura, including the timing of receipt of topline data from the GATHER2 clinical trial and its plans to file for marketing approval for geographic atrophy if the results of GATHER2 are positive, the potential utility of Zimura and the clinical meaningfulness of clinical trial results and data, including from post-hoc analyses of the GATHER1 clinical trial. Such forward-looking statements involve substantial risks and uncertainties that could cause the Company’s development programs, future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, those related to the progress and success of research and development programs and clinical trials, developments from the scientific and medical community and other factors discussed in the “Risk Factors” section contained in the quarterly and annual reports that the Company files with the
ISEE-G
View source version on businesswire.com: https://www.businesswire.com/news/home/20220715005442/en/
Investors:
Senior Vice President, Investor Relations
kathy.galante@ivericbio.com
or
Media:
Senior Director,
jeannie.neufeld@ivericbio.com
Source:







