Nanowear Receives FDA 510(k) Clearance for SimpleSENSE, a Cloth-Based Wearable Remote Diagnostic Monitoring Platform
Nanowear has received FDA Class II 510(k) clearance for SimpleSENSE, a groundbreaking cloth-based diagnostic platform. This multi-parameter remote diagnostic undergarment synchronously monitors cardiac, pulmonary, and circulatory health, capturing over 100 million data points daily. With the demand for telemedicine rising, SimpleSENSE offers clinicians a powerful tool for early patient intervention. As a response to the COVID-19 pandemic, Nanowear aims to assist in remote diagnostics across various healthcare areas. The company also plans to continue clinical trials related to heart failure and COVID-19.
- FDA Class II 510(k) clearance received for SimpleSENSE.
- SimpleSENSE captures over 100 million data points daily.
- Addresses increased demand for telemedicine and remote diagnostics.
- Plans to continue clinical trials for heart failure and COVID-19.
- None.
Insights
Analyzing...
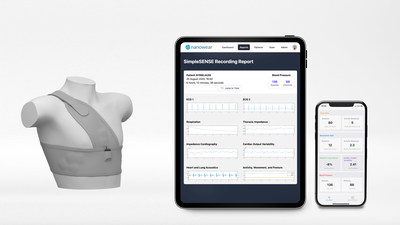
NEW YORK, Nov. 11, 2020 /PRNewswire/ -- Nanowear www.nanowearinc.com, the leading connected-care and remote diagnostic platform, today announced it has received FDA Class II 510(k) clearance for a first-of-its-kind cloth-based diagnostic platform, SimpleSENSE. SimpleSENSE is a multi-parameter remote diagnostic undergarment and machine learning digital platform, which simultaneously and synchronously monitors and assesses the heart, lungs, and upper vascular system.
Gender-neutral and size adjustable, the SimpleSENSE platform effectively replaces the digital stethoscope, multi-channel Holter monitor, Capnogram respiration machine, and blood pressure cuff by providing a diagnostic quality monitoring system that remotely captures more than 100 million data points per patient per day across cardiac, pulmonary, and circulatory biomarkers. With the increased need for telemedicine and remote diagnostic monitoring, SimpleSENSE provides a digital tool to assess medical data and trends between these biomarkers in a way that has not been previously available, empowering clinicians to treat patients earlier and more effectively.
"SimpleSENSE marks the company's second FDA 510(k) clearance and follows Nanowear's strategy of continued data-driven differentiation in the connected-care and remote diagnostic market," said Venk Varadan, co-founder and CEO of Nanowear. "In the face of the unexpected and unprecedented COVID-19 public health emergency, Nanowear began working collaboratively with FDA to evaluate a broadened indication for use for SimpleSENSE. Our platform can now efficiently serve the new need for remote diagnostics across primary care, acute illness and procedure, and chronic disease cases."
In addition to near-term commercialization of SimpleSENSE with select channel partners, Nanowear plans to continue its SimpleSENSE clinical trials in diagnosing worsening Heart Failure and COVID-19, maintaining a robust product and clinical R&D pipeline.
"Nanowear is transforming the virtual care continuum as physicians and hospitals can now safely monitor patients' multiple biomarkers and assess the associated medical trends indicative of clinical deterioration," said Spero Theodorou MD, Chief Medical Officer of InMode (NASDAQ: INMD). "The pandemic is transforming the way healthcare services are accessed and delivered today and for years to come. We are excited about the role that Nanowear will play in the field of remote diagnostics and how it will provide patients around the world access to better outcomes and improved quality of life, regardless of whether they are being cared for in the clinic or in the comfort of their homes."
For more information, please visit www.nanowearinc.com
About Nanowear
Nanowear is the leading developer of patented, cloth-based nanosensor technology with applications in the cardiac, neurological, industrial safety / government and sports medicine / performance diagnostics monitoring markets. The company's proprietary technology enables continuous and synchronous electrophysiological, hemodynamic, acoustic, metabolic and activity monitoring that empowers medical professionals with accurate diagnostic data through a cost effective and gender-neutral, size adjustable undergarment. Nanowear's core focus on innovation and next-gen technologies will continue to propel the company towards exploring unique and groundbreaking applications for its nanosensors. Headquartered in New York, NY, the company's research and development center is located in Berkeley, CA and University Park, PA.
Media Contact:
Dwain Schenck
(203) 223-5230
dwain@schenckstrategies.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/nanowear-receives-fda-510k-clearance-for-simplesense-a-cloth-based-wearable-remote-diagnostic-monitoring-platform-301171104.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/nanowear-receives-fda-510k-clearance-for-simplesense-a-cloth-based-wearable-remote-diagnostic-monitoring-platform-301171104.html
SOURCE Nanowear