Immatics Announces Multiple Presentations at the 39th Annual Meeting of the Society for Immunotherapy of Cancer (SITC) on TCR-T Therapy Candidates Targeting PRAME
Immatics (NASDAQ: IMTX) announced clinical data updates for its TCR-T therapy candidates targeting PRAME. The ACTengine® IMA203 demonstrated 54% confirmed objective response rate (cORR) and 12.1 months median duration of response in metastatic melanoma patients. The company plans to initiate SUPRAME, a Phase 3 trial, in December 2024. The next-generation IMA203CD8 showed enhanced pharmacology and potency, with the Phase 1a dose escalation study being reinitiated to target higher doses for solid cancers with medium-level PRAME copy numbers. The company expects to report initial clinical data from the TCER® IMA402 Phase 1a trial by year-end.
Immatics (NASDAQ: IMTX) ha annunciato aggiornamenti sui dati clinici per i suoi candidati alla terapia TCR-T che mirano a PRAME. L'ACTengine® IMA203 ha dimostrato un tasso di risposta obiettivo confermato (cORR) del 54% e una durata mediana della risposta di 12,1 mesi nei pazienti con melanoma metastatico. L'azienda prevede di avviare SUPRAME, uno studio di Fase 3, nel dicembre 2024. L'IMA203CD8 di nuova generazione ha mostrato una farmacologia e potenza migliorate, con lo studio di escalation della dose di Fase 1a che verrà riavviato per mirare a dosi più elevate per i tumori solidi con numeri di copie di PRAME a livello medio. L'azienda si aspetta di riportare i dati clinici iniziali dello studio di Fase 1a TCER® IMA402 entro la fine dell'anno.
Immatics (NASDAQ: IMTX) anunció actualizaciones de datos clínicos para sus candidatos a terapia TCR-T dirigidos a PRAME. El ACTengine® IMA203 demostró una tasa de respuesta objetiva confirmada (cORR) del 54% y una duración media de respuesta de 12,1 meses en pacientes con melanoma metastásico. La empresa planea iniciar SUPRAME, un ensayo de Fase 3, en diciembre de 2024. El IMA203CD8 de próxima generación mostró una farmacología y potencia mejoradas, con el estudio de escalado de dosis de Fase 1a que se reiniciará para apuntar a dosis más altas para cánceres sólidos con números de copias de PRAME en niveles medios. Se espera que la empresa informe datos clínicos iniciales del ensayo de Fase 1a TCER® IMA402 para finales de año.
임매틱스 (NASDAQ: IMTX)는 PRAME을 타겟으로 하는 TCR-T 치료 후보에 대한 임상 데이터 업데이트를 발표했습니다. ACTengine® IMA203은 전이성 흑색종 환자에서 54%의 확인된 객관적 반응률(cORR)과 12.1개월의 중간 반응 지속 시간을 보였습니다. 회사는 2024년 12월에 3상 시험인 SUPRAME를 시작할 계획입니다. 차세대 IMA203CD8은 향상된 약리학과 효능을 보여주었으며, 중간 수준의 PRAME 복제 수를 가진 고형암을 대상으로 더 높은 용량을 목표로 하는 1a상 용량 상승 연구를 재개할 예정입니다. 이 회사는 연말까지 TCER® IMA402 1a상 시험의 초기 임상 데이터를 보고할 것으로 예상하고 있습니다.
Immatics (NASDAQ: IMTX) a annoncé des mises à jour des données cliniques pour ses candidats à la thérapie TCR-T ciblant PRAME. L'ACTengine® IMA203 a démontré un taux de réponse objective confirmé (cORR) de 54% et une durée médiane de réponse de 12,1 mois chez les patients atteints de mélanome métastatique. L'entreprise prévoit de lancer SUPRAME, un essai de Phase 3, en décembre 2024. L'IMA203CD8 de nouvelle génération a montré une pharmacologie et une puissance améliorées, avec une étude d'escalade de dose de Phase 1a qui sera relancée pour cibler des doses plus élevées pour les cancers solides avec des taux moyens de copies de PRAME. L'entreprise prévoit de communiquer les premières données cliniques de l'essai de Phase 1a TCER® IMA402 d'ici la fin de l'année.
Immatics (NASDAQ: IMTX) hat klinische Datenaktualisierungen für seine TCR-T-Therapie-Kandidaten bekannt gegeben, die auf PRAME abzielen. Der ACTengine® IMA203 zeigte eine bestätigte objektive Reaktionsrate (cORR) von 54% und eine mediane Reaktionsdauer von 12,1 Monaten bei Patienten mit metastasierendem Melanom. Das Unternehmen plant, im Dezember 2024 SUPRAME, eine Phase-3-Studie, zu beginnen. Der nachfolgende IMA203CD8 zeigte verbesserte Pharmakologie und Potenz, wobei die Phase-1a-Dosissteigerungsstudie wieder aufgenommen wird, um höhere Dosen für solide Tumoren mit mittlerem PRAME-Kopienzahlen anzustreben. Das Unternehmen erwartet, bis zum Jahresende erste klinische Daten aus der Phase-1a-Studie TCER® IMA402 zu berichten.
- 54% confirmed objective response rate (cORR) in melanoma patients
- 12.1 months median duration of response
- Phase 3 SUPRAME trial planned for December 2024
- 41% objective response rate (ORR) for IMA203CD8
- 9.2 months median duration of response for IMA203CD8
- 84% tumor shrinkage rate in IMA203CD8 patients
- One Grade 5 adverse event possibly related to IMA203CD8 treatment
- Two patients experienced dose-limiting toxicities at dose level 4b
- Maximum tolerated dose not yet determined
Insights
The clinical data for IMA203 TCR-T therapy shows promising results with a
The next-generation IMA203CD8 demonstrates enhanced potency with a
This development positions Immatics strongly in the competitive cellular therapy landscape. The planned BLA submission in early 2027 provides a clear regulatory pathway. The expansion into multiple cancer types beyond melanoma, particularly those with medium-level PRAME expression, substantially increases the potential market opportunity. The comprehensive PRAME franchise strategy, including next-generation approaches with membrane-bound IL-15 and allogeneic options, creates multiple revenue opportunities and reduces clinical development risk through portfolio diversification.
Two oral presentations and multiple posters on clinical and
preclinical-stage candidates to be presented at SITC, demonstrating
the strength of Immatics’ TCR-T PRAME franchise to target solid cancers
- ACTengine® IMA203 demonstrates
54% cORR, 12.1 months mDOR and 6 months mPFS in heavily pretreated metastatic melanoma patients and >1-year mPFS in patients with deep responses; Company plans to start its randomized-controlled Phase 3 SUPRAME trial in December 2024 to evaluate IMA203 in second-line or later metastatic melanoma - Next-generation ACTengine® IMA203CD8 TCR-T cell therapy targeting PRAME demonstrates enhanced pharmacology and potency per cell; Phase 1a dose escalation reinitiated to target higher doses, positioning this TCR-T candidate for future development in solid cancers with medium-level PRAME copy numbers, such as ovarian cancer, endometrial cancers and triple-negative breast cancer
- A first update on Immatics’ Bispecific TCER® IMA402 targeting PRAME and initial clinical data from the ongoing Phase 1a dose escalation trial is expected to be reported by year-end
Houston, Texas and Tuebingen, Germany, November 8, 2024 – Immatics N.V. (NASDAQ: IMTX, “Immatics” or the “Company”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, today announced an expanded clinical dataset from the ongoing Phase 1b dose expansion clinical trial for ACTengine® IMA203 in addition to updated Phase 1 dose escalation clinical data on its next-generation ACTengine® IMA203CD8 TCR-T cell therapy. For the first time, the Company also reported preclinical data on other next-generation T cell candidates and combination strategies as part of its strategy to further exploit opportunities in additional solid tumor types within its PRAME franchise.
All dates and times of Immatics’ upcoming oral and poster presentations at the 39th Annual Meeting of the Society for Immunotherapy of Cancer (SITC) are available here. The data slides are accessible in the ‘Events & Presentations’ section of the Investor & Media section of the Company’s website.
“Immatics remains fully focused on the clinical development of our most advanced lead product candidate, IMA203, in second-line or later metastatic melanoma patients. We look forward to the initiation of SUPRAME, the registration-enabling Phase 3 trial, in December,” said Dr. Cedrik Britten, Chief Medical Officer at Immatics. “Today, we also provide an update on our first, next-generation cell therapy, IMA203CD8, which is designed to achieve enhanced anti-tumor activity. The data announced confirm IMA203CD8’s enhanced pharmacology and potency per cell in patients. These attributes highlight the potential of this therapy in hard-to-treat solid tumors with medium-level PRAME copy numbers, including ovarian, endometrial and triple-negative breast cancer. The next step will be to further increase the cell dose to assess the full clinical potential of IMA203CD8 beyond melanoma. In addition, we strive to continuously improve the potential therapeutic benefit for patients with a range of PRAME-positive cancers through the expansion of our PRAME franchise.”
ACTengine® IMA203 Monotherapy Phase 1b Trial - Clinical Data and Development Path Summary
On October 10, 2024, Immatics provided a data update on IMA203 monotherapy in 281 heavily pretreated metastatic melanoma patients from the ongoing Phase 1b dose expansion part of the clinical trial in which patients were treated at the recommended Phase 2 dose (RP2D, 1 to 10 billion total TCR-T cells).
The data announced today include all infused patients in the Phase 1b dose expansion part of the trial (N=412), consisting of the 28 melanoma patients reported on October 10, 2024, and 13 non-melanoma patients, of which 10 non-melanoma patients were reported on November 8, 2023.
IMA203 monotherapy has maintained a favorable tolerability profile with no treatment-related Grade 5 events in the entire safety population (N=703 Phase 1a and Phase 1b patients across all dose levels and all tumor types).
Best Overall Response for IMA203 in Dose Expansion in All Indications (N=41#)
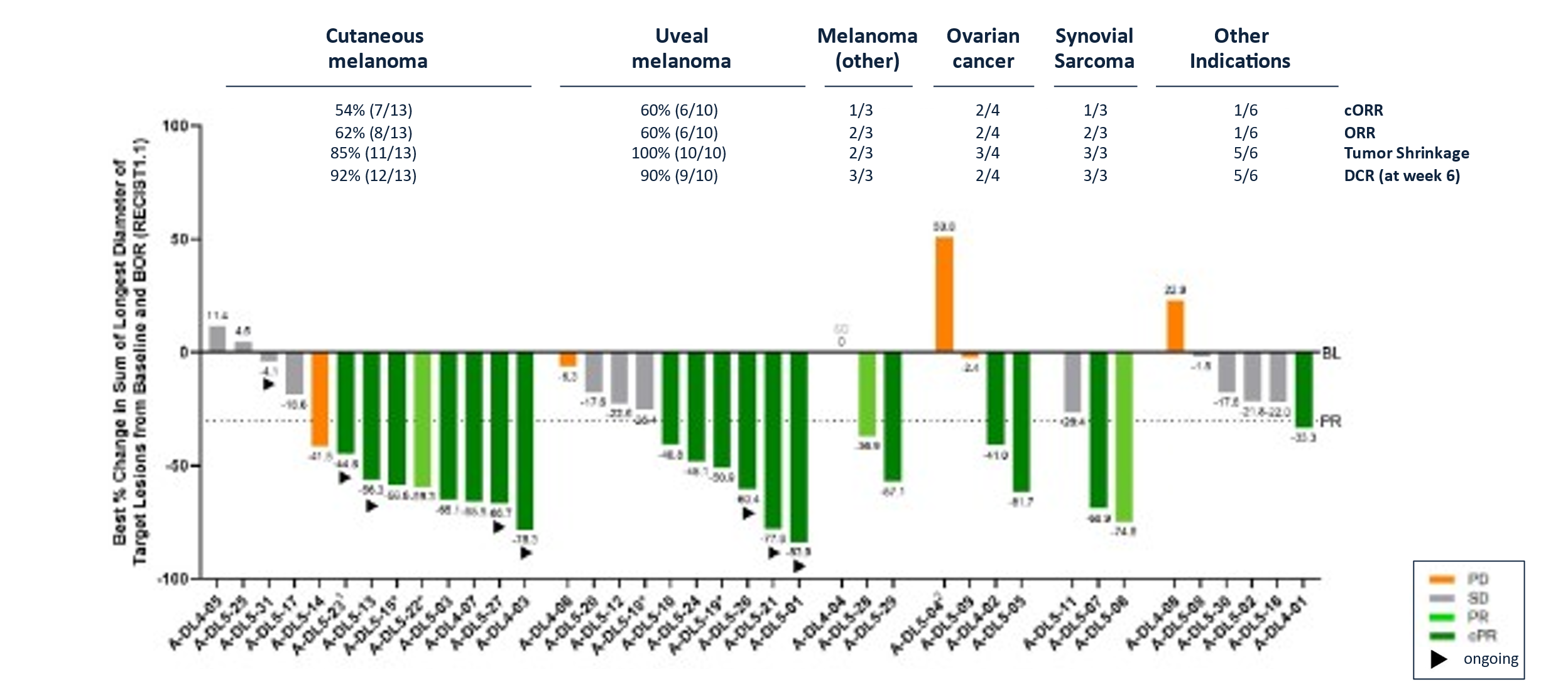
Data cut-off Aug 23, 2024; #First tumor assessment post infusion pending for 2/28 melanoma patients at data-cut; *Maximum change of target lesions and RECIST1.1 response at different timepoints. 1Patient A-DL5-23 is off study at data cut-off; 2Patient received one dose nivolumab erroneously.
Development Path for IMA203
Based on the Phase 1b data, the Company is on track to commence SUPRAME, the registration-enabling Phase 3 randomized-controlled clinical trial in melanoma for IMA203, in December 2024.
SUPRAME will evaluate IMA203 targeting PRAME in 360 HLA-A*02:01-positive patients with second-line or later (2L+) unresectable or metastatic melanoma who have received prior treatment with a checkpoint inhibitor. Patients will be randomized 1:1 for IMA203 or investigator’s choice of selected approved treatments in the 2L+ setting, including nivolumab/relatlimab, nivolumab, ipilimumab, pembrolizumab, lifileucel (US only) or chemotherapy. The primary endpoint for full approval will be median PFS and secondary endpoints will include objective response rate, safety, duration of response, no overall survival detriment and patient-reported outcomes.
Patient enrollment for SUPRAME is forecast to be completed in 2026, and a pre-specified interim analysis is planned for early 2026. Immatics aims to submit a Biologics License Application (BLA) in early 2027 for full approval.
ACTengine® IMA203CD8 (GEN2) Monotherapy Phase 1 Dose Escalation Trial - Patient Population & Clinical Data Summary
Patient population: Heavily pretreated patients with solid tumors
As of data cut-off on September 30, 2024, 444 heavily pretreated HLA-A*02:01 and PRAME-positive patients with solid tumors were infused with IMA203CD8 monotherapy across four escalating dose levels, of which 415 patients were evaluable for efficacy. The median total infused dose was 1.48x109 TCR-T cells, and the patient population is composed of patients with a median of three lines of prior systemic treatments.
Safety: Treatment with IMA203CD8 demonstrates a manageable tolerability profile across dose levels
IMA203CD8 monotherapy has maintained a manageable tolerability profile in the 44 patients treated. The most frequent adverse events at or above Grade 3 were expected cytopenia associated with lymphodepletion. Some patients also experienced mild to moderate CRS (Grade 1:
As previously reported, two patients experienced dose-limiting toxicities at dose level 4b, which prompted a dosing adjustment to dose level 4a. After further assessing the tolerability profile of IMA203CD8 in additional patients treated at dose level 4a, the eligibility criteria and the IL-2 dose regimen were modified, and dose escalation beyond dose level 4a was reinitiated. One Grade 5 adverse event classified as possibly related to treatment with IMA203CD8 was also observed as reported previously in March 2024. The maximum tolerated dose has not yet been determined.
Anti-tumor activity and durability: Deep and durable objective responses observed
- As of data cut-off on September 30, 2024, 10 of 17 responses were ongoing, of which three confirmed responses were ongoing at 14+, 15+ and 24+ months.
- Of note, these patients had been treated at substantially lower doses compared to IMA203 (GEN1), i.e. in a range of 0.2-0.48x109 TCR-T cells/m2 BSA (dose level 3) to 0.801-1.2x109 TCR-T cells/m2 BSA (dose level 4c) T cells infused.
- Deep responses with ≥
50% tumor size reduction were observed in 11 out of 17 responders. This group included two patients with complete response of target lesions, of which one patient showed a complete metabolic response according to PET-CT scan6. 41% (14/34) confirmed objective response rate (cORR) and41% (17/41) objective response rate (ORR).- Median duration of response (mDOR) of 9.2 months at a median follow-up (mFU) of 13.1 months.
- Tumor shrinkage7 of
84% (32/38) and disease control rate8 at week 6 of85% (34/40).
Translational data: Opportunity of IMA203CD8 in medium-level PRAME expressing indications
Translational data indicate that PRAME expression level is associated with clinical activity in IMA203 and IMA203CD8 treated patients. Both IMA203 and IMA203CD8 achieved deep responses despite IMA203CD8 patients receiving lower product doses. Based on the enhanced pharmacology of IMA203CD8, the evaluation of higher doses of IMA203CD8 in the ongoing dose escalation trial opens the possibility of addressing hard-to-treat solid tumor indications with a medium-level of PRAME copy numbers, such as ovarian cancer, endometrial cancers and triple-negative breast cancer.
Preclinical Data on New Approaches for TCR-T Based Cell Therapies
As part of Immatics’ long-term strategy to expand its PRAME franchise, the Company has conducted preclinical studies for the potential future clinical development of next-generation TCR-T-based cell therapies targeting PRAME to further enhance the efficacy and durability of IMA203. These efforts include the evaluation of TCR-T cells armored with membrane-bound IL-15 (mbIL15) targeting tumor types with low PRAME copy numbers, such as squamous non-small-cell lung cancer and squamous head and neck cancers. In addition, the Company is developing an allogeneic cell therapy approach to further increase commercial attractiveness and to reach patients quickly with its next-generation off-the-shelf cell therapy, ACTallo®. The preclinical data will be presented during poster sessions at SITC.
About ACTengine® IMA203, IMA203CD8 and Target PRAME
ACTengine® IMA203 is Immatics’ most advanced TCR-based autologous cell therapy that is directed against an HLA-A*02-presented (human leukocyte antigen) peptide derived from preferentially expressed antigen in melanoma (PRAME), a protein frequently expressed in a large variety of solid cancers. PRAME is homogeneously and specifically expressed in tumor tissue and Immatics’ PRAME peptide is present at a high copy number per tumor cell. The peptide has been identified and characterized by Immatics’ proprietary mass spectrometry-based target discovery platform, XPRESIDENT®. Through its proprietary TCR discovery and engineering platform XCEPTOR®, Immatics has generated a highly specific T cell receptor (TCR) against this target for ACTengine® IMA203.
ACTengine® IMA203 TCR-T is currently being evaluated as a monotherapy in a Phase 1 clinical trial in patients with solid tumors expressing PRAME, such as cutaneous melanoma. An IMA203 registration-enabling randomized controlled Phase 3 trial, “SUPRAME,” is planned to commence in December 2024.
ACTengine® IMA203 TCR-T is also currently being evaluated in Phase 1 IMA203CD8 (GEN2) monotherapy, where IMA203 engineered T cells are co-transduced with a CD8αβ co-receptor.
- END -
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
Immatics intends to use its website www.immatics.com as a means of disclosing material non-public information. For regular updates you can also follow us on X, Instagram and LinkedIn.
Forward-Looking Statements
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or the Company’s future financial or operating performance. For example, statements concerning timing of data read-outs for product candidates, the timing, outcome and design of clinical trials, the nature of clinical trials (including whether such clinical trials will be registration-enabling), the timing of IND or CTA filing for pre-clinical stage product candidates, the timing of BLA filings for clinical stage product candidates, estimated market opportunities of product candidates, the Company’s focus on partnerships to advance its strategy, and other metrics are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “plan”, “target”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management's control including general economic conditions and other risks, uncertainties and factors set forth in the Company’s Annual Report on Form 20-F and other filings with the Securities and Exchange Commission (SEC). Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. The Company undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
| Media | |
| Trophic Communications | |
| Phone: +49 171 3512733 | |
| immatics@trophic.eu |
| Immatics N.V. | |
| Jordan Silverstein | |
| Head of Strategy | |
| Phone: +1 346 319-3325 | |
| InvestorRelations@immatics.com |
1 Includes one patient who started lymphodepletion but did not receive IMA203 TCR-T cells.
2 All infused patients, first tumor assessment post infusion pending for 2/28 melanoma patients at data-cut.
3 All patients who started lymphodepletion as of the data cut-off on August 23, 2024.
4 All patients who started lymphodepletion.
5 All infused patients with at least one tumor assessment postbaseline.
6 Metabolic CR on investigator-initiated PET month 14 post infusion.
7 Three patients excluded from tumor shrinkage analysis and figures due to lack of post-treatment assessment.
8 One patient had an early tumor assessment, outside the first assessment visit window and is not included in DCR calculation.
Attachment








