Women Deemed Inoperable for Breast Cancer Benefitted from IceCure Medical's ProSense as an Independent Study Performed in Italy Showed a Tumor Reduction Rate of 93.43% to 96.81%
- The independent breast cancer study supports the safety and efficacy of IceCure's ProSense cryoablation system
- The study showed significant tumor size reduction rates: 21.59% for Luminal-A and 19.83% for Luminal-B at 1 month, 70.16% for Luminal-A and 60.71% for Luminal-B at 3 months, and 96.81% for Luminal-A and 93.43% for Luminal-B at 6 months
- In the Luminal-A group, there was an absence of residual malignant cells in all biopsy samples and a disappearance of lesions, indicating the complete effectiveness of the treatment
- None.
Insights
Analyzing...
- Disappearance of lesions and absence of residual malignant cells in Luminal-A group considered to be predictive for the complete effectiveness of the treatment
- This is the latest in a growing body of evidence presented by independent, non-sponsored doctors who use ProSense
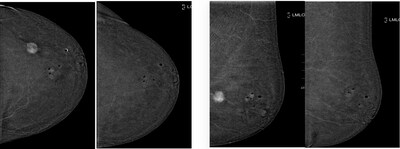
During the study, ultrasound-guided cryoablation using ProSense was performed on 28 women, who had biopsy-proven malignant lesions, and were deemed inoperable by a multidisciplinary group, and submitted to hormone therapy. At a median age of 73.4 years, 14 of the women were diagnosed with molecular subtype Luminal-A tumors and another 14 diagnosed with Luminal-B tumors. Patients were monitored at one, three, and six months post-procedure, at which time the tumor size reduction rate was evaluated by ultrasound. The effectiveness of the procedure was further evaluated after one year by core needle biopsy on the post-procedural scar (inside the breast at the site of the tumor) to determine the absence of residual tumoral cells. The size reduction rates were as follows:
1 month:
3 months:
6 months:
In the Luminal-A group, there was an absence of residual malignant cells in all biopsy samples and a disappearance of lesions, leading the study to conclude this is a predictive factor for the complete effectives of treatment. Luminal-A is the most common subtype and represents
"The patients in the study were deemed inoperable and had no treatment options available other than hormone therapy, and as we observe breast cancer awareness month, it is reassuring that a minimally invasive cryoablation procedure using IceCure's ProSense system is an available alternative," stated IceCure's CEO, Eyal Shamir. "This was a relatively short independent study that measured tumor size reduction, however the highly favorable results nonetheless validate similar results we are experiencing in our longer-term five-year post-procedure ICE3 study, which we expect to conclude in the first quarter of 2024. We believe the increasing number of doctors using ProSense and conducting these studies on their own initiative is the best testament to the usability and benefit of ProSense in real-world clinical settings."
The data from the
1. Yersal O, Barutca S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J Clin Oncol. 2014 Aug 10;5(3):412-24. doi: 10.5306/wjco.v5.i3.412. PMID: 25114856; PMCID: PMC4127612.
About IceCure Medical
IceCure Medical (Nasdaq: ICCM) develops and markets ProSense®, an advanced liquid-nitrogen-based cryoablation therapy for the treatment of tumors (benign and cancerous) by freezing, with the primary focus areas being breast, kidney, bone and lung cancer. Its minimally invasive technology is a safe and effective alternative to hospital surgical tumor removal that is easily performed in a relatively short procedure. The system is marketed and sold worldwide for the indications cleared and approved to date including in the
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995 and other Federal and Israeli securities laws. Words such as "expects," "anticipates," "intends," "plans," "believes," "seeks," "estimates" and similar expressions or variations of such words are intended to identify forward-looking statements. For example, IceCure is using forward looking statement in this press release when it discusses: the expected conclusion of its ICE3 study in the first quarter of 2024; and the belief that the increasing number of doctors using ProSense and conducting studies with ProSense is a testament to the usability and benefit of ProSense in real-world clinical settings. Historic results of scientific research, studies and clinical and preclinical trials do not guarantee that the conclusions of future research or trials will suggest identical or even similar conclusions. Because such statements deal with future events and are based on IceCure's current expectations, they are subject to various risks and uncertainties and actual results, performance, or achievements of IceCure could differ materially from those described in or implied by the statements in this press release. The forward-looking statements contained or implied in this press release are subject to other risks and uncertainties, many of which are beyond the control of the Company, including those set forth in the Risk Factors section of the Company's Annual Report on Form 20-F for the year ended December 31, 2022 filed with the SEC on March 29, 2023, and other documents filed with or furnished to the SEC which are available on the SEC's website, www.sec.gov. The Company undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
IR Contact:
Email: investors@icecure-medical.com
Michael Polyviou
Phone: 732-232-6914
Todd Kehrli
Phone: 310-625-4462
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/women-deemed-inoperable-for-breast-cancer-benefitted-from-icecure-medicals-prosense-as-an-independent-study-performed-in-italy-showed-a-tumor-reduction-rate-of-93-43-to-96-81-301948271.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/women-deemed-inoperable-for-breast-cancer-benefitted-from-icecure-medicals-prosense-as-an-independent-study-performed-in-italy-showed-a-tumor-reduction-rate-of-93-43-to-96-81-301948271.html
SOURCE IceCure Medical Ltd