Endocannabinoid Pathway Targeted Drug Demonstrates Proof-of-Concept Efficacy in a Cutaneous Lupus Erythematosus Mouse Model
Hoth (NASDAQ: HOTH) has partnered with Zylö Therapeutics to co-develop a topical treatment for Cutaneous Lupus Erythematosus (CLE), utilizing a patented delivery system called Z-pods™. The drug HT-005, targeting the endocannabinoid pathway, showed proof-of-concept efficacy in reducing skin plaques during a study in MRL/MpJ-Faslpr/J mice. This collaboration aims to address the limited therapeutic options available for CLE, which significantly impacts patient quality of life.
- Partnership with Zylö Therapeutics to co-develop HT-005 for CLE.
- HT-005 shows proof-of-concept efficacy in reducing skin plaques in animal models.
- Utilizes patented Z-pods™ for enhanced drug absorption.
- None.
Insights
Analyzing...
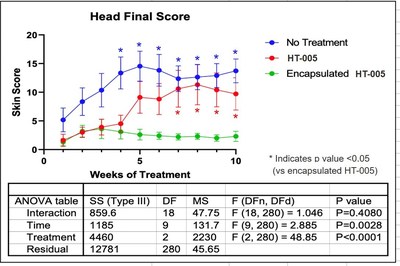
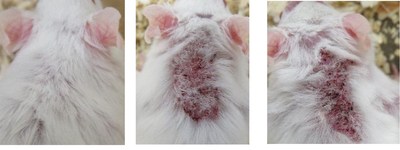
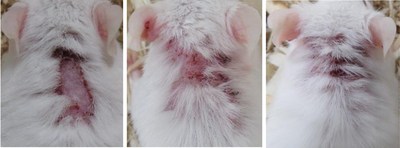
NEW YORK, Dec. 21, 2020 /PRNewswire/ -- Hoth (NASDAQ: HOTH) has a partnership agreement with Zylö Therapeutics Inc.,to co-develop a new topical treatment for patients with Cutaneous Lupus Erythematosus (CLE), a chronic autoimmune disease that affects the skin and is associated with a significant burden on patient quality of life. Therapeutic options for CLE are limited to steroids (topical and oral), topical calcineurin inhibitors, and other immunomodulating therapies that could have adverse effects during long-term use. Zylö Therapeutics has developed a patented topical delivery system using xerogel-derived nanoparticles called Z-pods™. Hoth has an exclusive license to develop HT-005, a drug that targets the endocannabinoid pathway, encapsulated in the Z-pods™ to enhance absorption into the skin. To investigate proof-of concept efficacy of HT-005 loaded Z-pods™, Zylö Therapeutics conducted a study in MRL/MpJ-Faslpr/J mice, an established animal model that shows symptoms similar to systemic lupus erythematosus (SLE) in humans. Results from the study demonstrated that the novel HT-005 loaded Z-pods™ were effective to reduce skin plaques associated with CLE, with statistical significance demonstrated in the overall average skin score as well as individual skin scores on the head and scapula.
Study Title: Topical treatment of cutaneous lesions in the MRL-Lpr mouse model of SLE
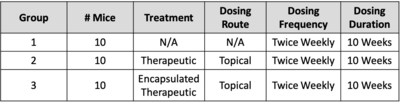
- 8 week old MRL/MpJ-Faslpr/J mice were randomly placed into a dosing group according to the Table below when an animal developed a skin plaque score of 1.
- Mice were treated for 10 weeks with either HT-005 alone or HT-005 encapsulated in Z-pods™ or not treated (control) HT-005 Z-Pods™
HT-005 Z-Pods™
Includes a novel topical delivery matrix (Z-Pods™) loaded with an endogenous ligand with suspected immune-modulating action. Z-Pods™ use patented xerogel-derived nanoparticles for sustained and controlled topical delivery of active pharmaceutical ingredients.
Partnership
The study was conducted at The Jackson Laboratory
Profile
Hoth Therapeutics, Inc. is a clinical-stage biopharmaceutical company focused on developing new generation therapies for dermatological disorders. Hoth's mission is to develop novel therapies that improve patient quality of life. Hoth has exclusive worldwide rights to the BioLexa Platform.
Company Contact
HOTH THERAPEUTICS
One Rockefeller Plaza, Suite 1039
New York, New York 10020
LR Advisors LLC
investorrelations@hoththerapeutics.com
www.hoththerapeutics.com
Phone: (678) 570-6791
Safe Harbor Statement: This document contains "forward-looking statements" within the meaning of the "safe-harbor" provisions of the Private Securities Litigation Reform Act of 1995. These statements are identified by the use of words "could", "believe", "anticipate", "intend", "estimate", "expect", "may", "continue", "predict", "potential" and similar expressions that are intended to identify forward-looking statements. Such statements involve known and unknown risks, uncertainties and other factors that could cause the actual results of Hoth Therapeutics, Inc. ("Hoth" or "the Company") to differ materially from the results expressed or implied by such statements, including changes to anticipated sources of revenues, future economic and competitive conditions, difficulties in developing the Company's technology platforms, retaining and expanding the Company's customer base, fluctuations in consumer spending on the Company's products and other factors. Accordingly, although the Company believes that the expectations reflected in such forward-looking statements are reasonable, there can be no assurance that such expectations will prove to be correct. The Company disclaims any obligations to publicly update or release any revisions to the forward- looking information contained in this document, whether as a result of new information, future events or otherwise, after the date of this document or to reflect the occurrence of unanticipated events except as required by law.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/endocannabinoid-pathway-targeted-drug-demonstrates-proof-of-concept-efficacy-in-a-cutaneous-lupus-erythematosus-mouse-model-301196588.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/endocannabinoid-pathway-targeted-drug-demonstrates-proof-of-concept-efficacy-in-a-cutaneous-lupus-erythematosus-mouse-model-301196588.html
SOURCE Hoth Therapeutics, Inc.