NovaSure® V5 Global Endometrial Ablation Device Approved for Use in Canada and Europe
Hologic Inc. announced the approval of its NovaSure® V5 global endometrial ablation device in Canada and Europe. This advanced version features enhanced technology aimed at treating diverse cervical and uterine anatomies, enhancing patient outcomes. With over 3 million women treated globally, the NovaSure V5 offers a 97% patient satisfaction rate and an 87% hysterectomy avoidance rate at ten years post-procedure. Key improvements include an updated cervical seal and enhanced accuracy markings. The company emphasizes ongoing commitment to innovation in women's health.
- Approval of NovaSure V5 in Canada and Europe enhances market reach.
- NovaSure device has treated over 3 million patients, demonstrating strong acceptance in the market.
- 97% patient satisfaction and 87% of patients avoiding hysterectomy at 10 years shows significant efficacy.
- None.
Insights
Analyzing...
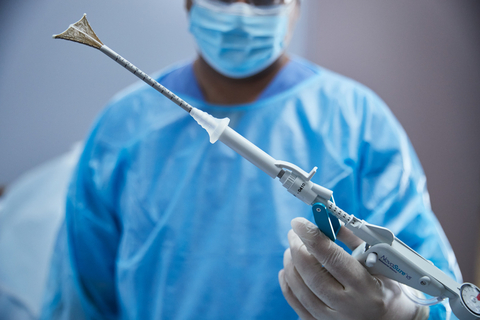
NovaSure® V5 Global Endometrial Ablation Device Approved for Use in
“Since its launch over 20 years ago, we have constantly listened to our customers’ feedback, which has driven continuous design improvements for NovaSure,” said
NovaSure V5 has an updated cervical seal, featuring EndoForm™ technology, designed to increase the sealing surface and accommodate a range of cervical canals and anatomical variability.1,2,3 The device’s AccuSheath™ markings are designed to improve the accuracy and confidence of seating and fundal placement.4 Additionally, NovaSure V5 is equipped with SureClear™ technology, Hologic's unique fluid removal system, which provides integrated suction through the array by constant tissue contact while simultaneously removing ablation byproducts such as vapor and fluid.5
“I welcome the new features on the NovaSure V5. These will support both inexperienced and more established users in performing effective, more efficient and accurate procedures,” said Dr.
The NovaSure device enables an endometrial ablation procedure for the treatment of abnormal uterine bleeding, with
For more information about the benefits and risks of NovaSure V5, visit: www.gynsurgicalsolutions.co.uk/NovaSureV5.
About Hologic
The company also champions women through the Hologic Global Women’s Health Index, which provides a science-backed data roadmap for improving women’s well-being, and Project Health Equality, which elevates awareness, research insights and access to quality care for underserved women.
Forward-Looking Statements
This press release may contain forward-looking information that involves risks and uncertainties, including statements about the use of Hologic's NovaSure system. There can be no assurance this product will achieve the benefits described herein or that such benefits will be replicated in any particular manner with respect to an individual patient. The actual effect of the use of the product can only be determined on a case-by-case basis depending on the particular circumstances and patient in question. In addition, there can be no assurance that this product will be commercially successful or achieve any expected level of sales. Hologic expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any such statements presented herein to reflect any change in expectations or any change in events, conditions or circumstances on which any such statements are based.
This information is not intended as a product solicitation or promotion where such activities are prohibited. For specific information on what products are available for sale in a particular country, please contact a local Hologic sales representative or write to womenshealth@hologic.com.
Hologic, AccuSheath, EndoForm, NovaSure, SureClear and The Science of Sure are trademarks and/or registered trademarks of
Notes and Disclaimers
IMPORTANT SAFETY INFORMATION: NovaSure endometrial ablation is for premenopausal women with heavy periods due to benign causes who are finished childbearing. Pregnancy following the NovaSure procedure can be dangerous. The NovaSure procedure is not for those who have or suspect uterine cancer; have an active genital, urinary or pelvic infection; or have an IUD. NovaSure endometrial ablation is not a sterilization procedure. Rare but serious risks include, but are not limited to, thermal injury, perforation and infection. Temporary side effects may include cramping, nausea, vomiting, discharge and spotting. Inform patients to contact you if they experience a possible side effect related to use of this product. For detailed benefit and risk information, please visit www.gynsurgicalsolutions.co.uk/NovaSureV5.
SOURCE:
References
1 NovaSure V5 cervical seal drawing, FAB-18821
2 Baggish, MS, Karram, MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 4th edition.
3 Luo, J et al. Quantitative analyses of variability in normal vaginal shape and dimension on MR images. Int. Urogynecol (2016) 27:1087-1095
4 NovaSure V5 Designs Verification Results, VER-10513
5 NovaSure V5 Instructions for Use, MAN-07653-001
6 Baskett TF, Clough H, Scott TA. NovaSure bipolar radiofrequency endometrial ablation: report of 200 cases.
7 Herman M, Penninx J, Mol B, Bongers M. Ten-year follow-up of a randomised controlled trial comparing bipolar endometrial ablation with balloon ablation for heavy menstrual bleeding. BJOG 2013;120:966–970
View source version on businesswire.com: https://www.businesswire.com/news/home/20230215005183/en/
Media Contact:
Vice President, Communications
(+1) 508.263.8764
jane.mazur@hologic.com
Investor Contact:
Vice President, Investor Relations
(+1) 858.410.8514
ryan.simon@hologic.com
Source:







