Hologic to Launch the NovaSure® V5 Global Endometrial Ablation Device at Upcoming American Association of Gynecologic Laparoscopists 50th Global Congress
Hologic, Inc. (NASDAQ: HOLX) is set to launch the NovaSure® V5 global endometrial ablation device at the AAGL 50th Global Congress (November 14-17). This innovative device, building on a 20-year legacy of the NovaSure system, aims to enhance the physician experience. With over 3 million treatments performed since its FDA approval in 2001, the device incorporates advanced features like the EndoForm™ cervical seal technology and SureClear™ fluid removal system. The launch reflects Hologic's commitment to improving outcomes for women and providing physicians with reliable tools.
- None.
- None.
Insights
Analyzing...
With a 20-Year Legacy, the NovaSure System is the Most Trusted in Global Endometrial Ablation to Address Abnormal Uterine Bleeding (AUB)
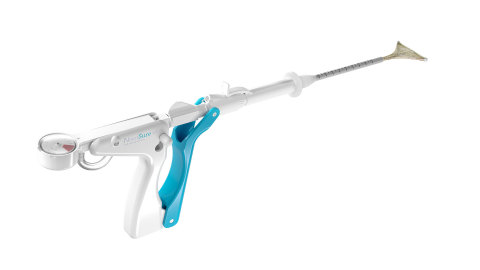
NovaSure V5 (Photo: Business Wire)
Over the last 20 years, the NovaSure system has built a trusted legacy in GEA. The NovaSure device first received FDA approval in 2001, and by 2018 the NovaSure procedure had been used to treat over 3 million patients.2
“The NovaSure V5 device is at the forefront of innovation, established on an unmatched 20-year history of success and updated based on customer feedback,” says
Guided by women’s unique needs and the physicians that treat them, the NovaSure V5 device features several innovations, making it the most sophisticated device in the NovaSure family. The device has an updated cervical seal, featuring EndoForm™ technology, designed to increase the sealing surface and accommodate a range of cervical canals and anatomical variability.3,4,5 The device’s AccuSheath™ markings are designed to improve the accuracy and confidence of seating and fundal placement.6 Additionally, the NovaSure V5 device is equipped with SureClear™ technology, Hologic's unique fluid removal system, which provides integrated suction through the array by constant tissue contact while simultaneously removing ablation byproducts such as vapor and fluid from the uterus.7
"I have used NovaSure for 20 years and consider it the gold standard treatment for patients with menstrual disorders. Believing there was no needed room for improvement, the V5 enhanced cervical seal feature has exceeded my expectations,” says Dr.
The NovaSure V5 endometrial ablation system is not only outfitted to support the physician through comfort and control but is also specifically designed to cater to various cervical canal sizes, staying true to Hologic's promise of keeping the patient in the forefront of every decision.3
Learn more about the advancements made to the innovative NovaSure V5 device and the impact these changes will have on physician experience at the AAGL hybrid conference. The NovaSure V5 device will be on display at the Hologic booth #536 and online at Hologic’s virtual surgery suite (https://hologic.protoexpo.com/surgical/).
For more information about the benefits and risks of the NovaSure V5 device, visit: https://gynsurgicalsolutions.com/hcp/hysteroscopy/novasure-endometrial-ablation/.
About Hologic
Forward-Looking Statements
This press release may contain forward-looking information that involves risks and uncertainties, including statements about the use of Hologic's NovaSure system. There can be no assurance this product will achieve the benefits described herein or that such benefits will be replicated in any particular manner with respect to an individual patient. The actual effect of the use of the product can only be determined on a case-by-case basis depending on the particular circumstances and patient in question. In addition, there can be no assurance that this product will be commercially successful or achieve any expected level of sales. Hologic expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any such statements presented herein to reflect any change in expectations or any change in events, conditions, or circumstances on which any such statements are based.
Hologic, AccuSheath, EndoForm, NovaSure, SureClear and The Science of Sure are trademarks and/or registered trademarks of
Sources:
-
U.S Market Report Suite for Gynecological Devices. iData
Research Inc. , 2020. -
Hologic, Inc. Data on file; 2004-2018. Based on units shipped from 2004-2018. - NovaSure V5 cervical seal drawing, FAB-18821
-
Baggish, M.S., Karram, M/M., Atlas of Pelvic Anatomy and Gynecologic Surgery. 4th edition.
Elsevier 2016. ISBN: 978-0323225526. Chapter 44, Page 493. - Luo, J. et al. Quantitative analyses of variability in normal vaginal shape and dimension on MR images. Int. Urogynecol (2016) 27:1087-1095
- NovaSure V5 Designs Verification Results, VER-10513
- NovaSure V5 Instructions for Use, MAN-07653-001
Notes and Disclaimers
IMPORTANT SAFETY INFORMATION NovaSure® endometrial ablation is for premenopausal women with heavy periods due to benign causes who are finished childbearing. Pregnancy following the NovaSure procedure can be dangerous. The NovaSure procedure is not for those who have or suspect uterine cancer; have an active genital, urinary or pelvic infection; or an IUD. NovaSure endometrial ablation is not a sterilization procedure. Rare but serious risks include, but are not limited to, thermal injury, perforation and infection. Temporary side effects may include cramping, nausea, vomiting, discharge and spotting. Inform patients to contact you if they experience a possible side effect related to use of this product. For detailed benefit and risk information, please consult the IFU.
SOURCE
View source version on businesswire.com: https://www.businesswire.com/news/home/20211102005175/en/
Media Contact:
Vice President, Divisional Communications
(508) 263-8764
Investor Contacts:
Vice President, Investor Relations and Corporate Communications
(858) 410-8588
Vice President, Investor Relations
(858) 410-8514
Source:







