Phase III RUBY clinical trial demonstrates potential of Jemperli (dostarlimab-gxly) plus chemotherapy to redefine the treatment of primary advanced or recurrent endometrial cancer versus chemotherapy alone
GSK announced promising interim results from the RUBY phase III trial, which evaluates dostarlimab-gxly combined with standard chemotherapy for advanced endometrial cancer. Notably, a 72% reduction in disease progression risk was observed in the dMMR/MSI-H subgroup, while an overall reduction of 36% was reported across all participants. The trial showcased clinically meaningful trends in overall survival and was presented at prestigious medical forums, with regulatory submissions anticipated by mid-2023. These advancements underscore GSK’s commitment to improving treatment options for endometrial cancer.
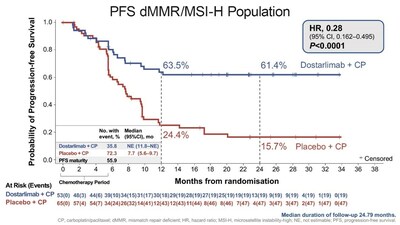
- 72% reduction in disease progression risk in dMMR/MSI-H population.
- 36% reduction in risk of disease progression or death in overall population.
- Clinically meaningful overall survival trends observed.
- Regulatory submissions planned for the first half of 2023.
- Statistical significance for overall survival not reached at interim analysis.
Insights
Analyzing...
72% and36% reduction in the risk of disease progression or death observed in the dMMR/MSI-H population and overall patient population, respectively- Clinically meaningful overall survival trend observed at interim analysis
- Results presented in same-day presentations at ESMO Virtual Plenary and SGO Annual Meeting and simultaneously published in The
New England Journal of Medicine - Regulatory submissions planned for the first half of 2023
A statistically significant and clinically meaningful improvement in progression free survival (PFS) was observed for dostarlimab-gxly plus carboplatin/paclitaxel in the mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) population (n=118) and in the overall population (n=494) versus placebo plus chemotherapy. The separation of the lines in the accompanying Kaplan-Meier curve illustrate the significant reduction in risk of disease progression or death in patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer in the dostarlimab plus chemotherapy treatment arm compared to the placebo plus chemotherapy treatment arm.
Dr.
Additionally, at this first interim analysis, there was a clinically meaningful overall survival trend in the overall population among patients receiving dostarlimab-gxly plus chemotherapy followed by dostarlimab-gxly. The analysis was done at
dostarlimab-gxly + | placebo + chemotherapy | |
PFS dMMR/MSI-H population | ||
Number of patients evaluated | 53 | 65 |
HR ( | 0.28 (0.162-0.495) | |
P-value | P<0.0001 | |
At 24 months ( | (46.3–73.4) | (7.2–27.0) |
PFS overall patient population | ||
Number of patients evaluated | 245 | 249 |
HR ( | 0.64 | |
P-value | P<0.0001 | |
At 24 months ( | ( | ( |
PFS MMRp/MSS populationa | ||
Number of patients evaluated | 192 | 184 |
HR ( | 0.76 | |
P-value | N/A | |
At 24 months ( | (21.2–36.0) | (12.8–25.7) |
OS overall patient populationb | ||
HR ( | 0.64 | |
P-value | P=0.0021c
| |
At 24 months ( | (64.5–77.1) |
|
OS dMMR/MSI-H populationa,d | ||
HR ( | 0.30 (0.127–0.699) | |
P-value | N/A | |
At 24 months ( | (66.8–92.0) | (43.4–71.2) |
OS MMRp/MSS populationa,e | ||
HR ( | 0.73 | |
P-value | N/A | |
At 24 months ( | (59.8–74.4) | (46.8–62.5) |
aExploratory analyses of PFS in MMRp/MSS, OS in dMMR/MSI-H, and OS in MMRp/MSS populations were pre-specified with no planned hypothesis testing. bMaturity ≈ |
The safety and tolerability profile of dostarlimab-gxly in combination with carboplatin/paclitaxel in the RUBY phase III trial was generally consistent with the known safety profiles of the individual agents. The most common (>
These data were shared in a
RUBY is part of an international collaboration between the European Network of Gynaecological Oncological Trial groups (ENGOT), a research network of the
GSK's ambition is for dostarlimab-gxly to become the backbone of the Company's ongoing immuno-oncology-based research and development program when used alone and in combination with standard of care and future novel cancer therapies, particularly for patients who currently have limited treatment options. Dostarlimab-gxly is being investigated in registrational enabling studies as monotherapy and as part of combination regimens, including in patients with primary advanced or recurrent endometrial cancer, patients with Stage III or IV non-mucinous epithelial ovarian cancer, and patients with other advanced solid tumors or metastatic cancers.
About endometrial cancer
Endometrial cancer is found in the inner lining of the uterus, known as the endometrium. Endometrial cancer is the most common gynecologic cancer in developed countries, with approximately 417,000 new cases reported each year worldwide[i], and incidence rates are expected to rise by almost
About RUBY
RUBY is a two-part global, randomized, double-blind, multicenter phase III trial of patients with primary advanced or recurrent endometrial cancer. Part 1 is evaluating dostarlimab-gxly plus carboplatin-paclitaxel followed by dostarlimab-gxly versus carboplatin-paclitaxel plus placebo followed by placebo. Part 2 is evaluating dostarlimab-gxly plus carboplatin-paclitaxel followed by dostarlimab-gxly plus niraparib versus placebo plus carboplatin-paclitaxel followed by placebo. The primary endpoints in Part 1 are investigator-assessed PFS based on the Response Evaluation Criteria in Solid Tumors v1.1 and OS. The statistical analysis plan included pre-specified analyses of PFS in the dMMR/MSI-H and ITT populations and OS in the overall population. Pre-specified exploratory analyses of PFS in the MMRp/MSS population and OS in the dMMR/MSI-H populations were also performed. RUBY Part 1 included a broad population, including histologies often excluded from clinical trials and had approximately
About Jemperli (dostarlimab-gxly)
Jemperli is a programmed death receptor-1 (PD-1)-blocking antibody that binds to the PD-1 receptor and blocks its interaction with the PD-1 ligands PD-L1 and PD-L2.[v]
Jemperli is not approved anywhere in the world for use in combination with standard-of-care chemotherapy (carboplatin-paclitaxel) followed by dostarlimab-gxly for primary advanced or recurrent endometrial cancer.
Jemperli was discovered by AnaptysBio, Inc. and licensed to
Indications and Important Safety Information for JEMPERLI (dostarlimab-gxly)
JEMPERLI is indicated for the treatment of adult patients with mismatch repair deficient (dMMR) recurrent or advanced:
- endometrial cancer (EC), as determined by an FDA-approved test, that has progressed on or following prior treatment with a platinum-containing regimen in any setting and are not candidates for curative surgery or radiation, or
- solid tumors, as determined by an FDA-approved test, that have progressed on or following prior treatment and who have no satisfactory alternative treatment options. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Important Safety Information
Severe and Fatal Immune-Mediated Adverse Reactions
- Immune-mediated adverse reactions, which can be severe or fatal, can occur in any organ system or tissue and can occur at any time during or after treatment with a PD-1/PD-L1–blocking antibody, including JEMPERLI.
- Monitor closely for signs and symptoms of immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function tests at baseline and periodically during treatment. For suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
- Based on the severity of the adverse reaction, withhold or permanently discontinue JEMPERLI. In general, if JEMPERLI requires interruption or discontinuation, administer systemic corticosteroids (1 to 2 mg/kg/day prednisone or equivalent) until improvement to ≤Grade 1. Upon improvement to ≤Grade 1, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reaction is not controlled with corticosteroids.
Immune-Mediated Pneumonitis
- JEMPERLI can cause immune-mediated pneumonitis, which can be fatal. In patients treated with other PD-1/PD-L1–blocking antibodies, the incidence of pneumonitis is higher in patients who have received prior thoracic radiation. Pneumonitis occurred in
2.3% (14/605) of patients, including Grade 2 (1.3% ), Grade 3 (0.8% ), and Grade 4 (0.2% ) pneumonitis.
Immune-Mediated Colitis
- Colitis occurred in
1.3% (8/605) of patients, including Grade 2 (0.7% ) and Grade 3 (0.7% ) adverse reactions. Cytomegalovirus infection/reactivation have occurred in patients with corticosteroid-refractory immune-mediated colitis. In such cases, consider repeating infectious workup to exclude alternative etiologies.
Immune-Mediated Hepatitis
- JEMPERLI can cause immune-mediated hepatitis, which can be fatal. Grade 3 hepatitis occurred in
0.5% (3/605) of patients.
Immune-Mediated Endocrinopathies
- Adrenal Insufficiency
- Adrenal insufficiency occurred in
1.2% (7/605) of patients, including Grade 2 (0.5% ) and Grade 3 (0.7% ). For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment per institutional guidelines, including hormone replacement as clinically indicated. Withhold or permanently discontinue JEMPERLI depending on severity. - Hypophysitis
- JEMPERLI can cause immune-mediated hypophysitis. Grade 2 hypophysitis occurred in
0.2% (1/605) of patients. Initiate hormone replacement as clinically indicated. Withhold or permanently discontinue JEMPERLI depending on severity. - Thyroid Disorders
- Grade 2 thyroiditis occurred in
0.5% (3/605) of patients. Grade 2 hypothyroidism occurred in7.6% (46/605) of patients. Hyperthyroidism occurred in2.3% (14/605) of patients, including Grade 2 (2.1% ) and Grade 3 (0.2% ). Initiate hormone replacement or medical management of hyperthyroidism as clinically indicated. Withhold or permanently discontinue JEMPERLI depending on severity. - Type 1 Diabetes Mellitus, Which Can Present with Diabetic Ketoacidosis
- JEMPERLI can cause type 1 diabetes mellitus, which can present with diabetic ketoacidosis. Grade 3 type 1 diabetes mellitus occurred in
0.2% (1/605) of patients. Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold or permanently discontinue JEMPERLI depending on severity.
Immune-Mediated Nephritis with Renal Dysfunction
- JEMPERLI can cause immune-mediated nephritis, which can be fatal. Grade 2 nephritis, including tubulointerstitial nephritis, occurred in
0.5% (3/605) of patients.
Immune-Mediated Dermatologic Adverse Reactions
- JEMPERLI can cause immune-mediated rash or dermatitis. Bullous and exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS), have occurred with PD-1/PD-L1–blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-bullous/exfoliative rashes. Withhold or permanently discontinue JEMPERLI depending on severity.
Other Immune-Mediated Adverse Reactions
- The following clinically significant immune-mediated adverse reactions occurred in <
1% of the 605 patients treated with JEMPERLI or were reported with the use of other PD-1/PD-L1–blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions. - Nervous System: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis, Guillain-Barre syndrome, nerve paresis, autoimmune neuropathy
- Cardiac/Vascular: Myocarditis, pericarditis, vasculitis
- Ocular: Uveitis, iritis, other ocular inflammatory toxicities. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur
- Gastrointestinal: Pancreatitis, including increases in serum amylase and lipase levels, gastritis, duodenitis
- Musculoskeletal and Connective Tissue: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatica
- Endocrine: Hypoparathyroidism
- Other (Hematologic/Immune): Autoimmune hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection
Infusion-Related Reactions
- Severe or life-threatening infusion-related reactions have been reported with PD-1/PD-L1–blocking antibodies. Severe infusion-related reactions (Grade 3) occurred in
0.2% (1/605) of patients receiving JEMPERLI. Monitor patients for signs and symptoms of infusion-related reactions. Interrupt or slow the rate of infusion or permanently discontinue JEMPERLI based on severity of reaction.
Complications of Allogeneic HSCT
- Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after treatment with a PD-1/PD-L1–blocking antibody, which may occur despite intervening therapy. Monitor patients closely for transplant-related complications and intervene promptly.
Embryo-Fetal Toxicity and Lactation
- Based on its mechanism of action, JEMPERLI can cause fetal harm. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with JEMPERLI and for 4 months after their last dose. Because of the potential for serious adverse reactions from JEMPERLI in a breastfed child, advise women not to breastfeed during treatment with JEMPERLI and for 4 months after their last dose.
Common Adverse Reactions
The most common adverse reactions (≥
The most common adverse reactions (≥
Please see the full US Prescribing Information.
GSK in oncology
GSK is committed to maximizing patient survival through transformational medicines. GSK's pipeline is focused on immuno-oncology, tumor cell targeting therapies and synthetic lethality. Our goal is to achieve a sustainable flow of new treatments based on a diversified portfolio of investigational medicines utilizing modalities such as small molecules, antibodies, and antibody-drug conjugates, either alone or in combination.
About GSK
GSK is a global biopharma company with a purpose to unite science, technology, and talent to get ahead of disease together. Find out more at us.gsk.com/en-us/company
GSK inquiries | |||
Media: | +44 (0) 20 8047 5502 | ( | |
+44 (0) 20 8047 5502 | ( | ||
+1 202 603 5003 | ( | ||
+1 202 302 4595 | ( | ||
Investor Relations: | +44 (0) 7717 618834 | ( | |
+44 (0) 20 8047 2406 | ( | ||
+44 (0) 7990 339653 | ( | ||
+44 (0) 7385 415719 | ( | ||
+44 (0) 7803 050238 | ( | ||
+44 (0) 7796 707505 | ( | ||
+1 215 751 7002 | ( | ||
+1 215 751 4855 | ( |
Cautionary statement regarding forward-looking statements
GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Such factors include, but are not limited to, those described under Item 3.D 'Risk factors" in the company's Annual Report on Form 20-F for 2022, GSK's Q4 Results for 2022 and any impacts of the COVID-19 pandemic.
Registered in
No. 3888792
Registered Office:
Brentford,
TW8 9GS
References
i Faizan U, Muppidi V. Uterine Cancer. [Updated 2022
ii Braun MM, et al. Am Fam Physician. 2016;93(6):468-474.
iii
iv Kantar Health, Cust Study (2018).
v Laken H, Kehry M, Mcneeley P, et al. Identification and characterization of TSR-042, a novel anti-human PD-1 therapeutic antibody.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/phase-iii-ruby-clinical-trial-demonstrates-potential-of-jemperli-dostarlimab-gxly-plus-chemotherapy-to-redefine-the-treatment-of-primary-advanced-or-recurrent-endometrial-cancer-versus-chemotherapy-alone-301782647.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/phase-iii-ruby-clinical-trial-demonstrates-potential-of-jemperli-dostarlimab-gxly-plus-chemotherapy-to-redefine-the-treatment-of-primary-advanced-or-recurrent-endometrial-cancer-versus-chemotherapy-alone-301782647.html
SOURCE