Paragon 28 Launches the R3FLEX™ Stabilization System to Anatomically Repair Ankle Syndesmotic Injuries
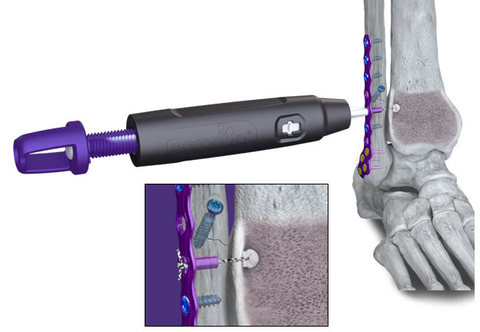
Figure 1: R3FLEX™ Stabilization System and Gorilla® Ankle Fracture Plating System used to treat an ankle fracture with syndesmotic injury. (Graphic: Business Wire)
Ankle fractures are one of the most common injuries with over 470,000 predicted in 2024 in the US.1,2 Injury to the distal tibiofibular syndesmosis is estimated to occur in
The R3FLEX™ Stabilization System gives surgeons the ability to precisely adjust and visualize tension during the repair as needed with a simple turn of the handle. The design and functionality of the device allows surgeons to restore patients’ soft tissue to a more natural anatomy, helping to mitigate arthritic response and minimize the risk of hardware removal.
During preparation and implantation of the R3FLEX™ Implant, the tibial implant rests on the internal surface of the tibia rather than external to the cortical bone. Screws and other flexible fixation products may require hardware on the external surface of the tibial bone, which may increase the risk of injury to the saphenous nerve and great saphenous vein during preparation and implantation.6
The implant’s innovative design also utilizes a short suture loop between the all-suture anchor in the tibia and the titanium fibular component to create a strong repair construct that is less susceptible to elongation or creep that may cause concern for some surgeons.7 The fibular component contains a thermoplastic urethane bumper inside to allow for micromotion in the repair so the fibula can move in a controlled manner similar to its uninjured state.
The R3FLEX™ Stabilization System is delivered to surgeons in a single sterile kit that includes both the implants and instruments to simplify surgical workflow and inventory management.
Paragon 28’s CEO, Albert DaCosta, commented, “The R3FLEX Stabilization System is the crown jewel of our syndesmotic injury repair portfolio with novel features that give surgeons the ability, for the first time, to precisely adjust and visualize tension during a repair with the simple turn of the handle. Dynamic tensioning is incredibly important, and we believe it will help mitigate arthritic response, which is a primary complication following these types of ankle fractures.”
Surgeon Designer Lauren Geaney, MD, commented, “R3FLEX is an exciting solution to syndesmotic fixation: providing improved and reliable stability while simultaneously allowing forgiveness to malreduction. Additionally, the fixation on the lateral tibial cortex decreases potential for interference with medial tibial fracture hardware or injury to medial distal tibial neurovasculature.”
The R3FLEX™ Stabilization System bolsters Paragon 28’s soft tissue offering, which includes the Grappler® Suture Anchor System, Grappler® Knotless Anchor System, Bridgeline™ Tape, Grappler® Interference Screw System, Grappler® R3INFORCE™ Extraosseous Repair System, R3ACT® Stabilization System, R3LEASE™ Stabilization System, Paratrooper™ Plantar Plate System, TenoTac® 2.0 Soft Tissue Fixation System, and Mister Tendon™ Harvester System. With this comprehensive portfolio, Paragon 28® provides its customers with a single source to address their foot and ankle soft tissue needs.
About Paragon 28, Inc.
Based in
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward-looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward-looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K/A for the fiscal year ended December 31, 2023 and its Quarterly Reports on Form 10-Q, as updated periodically with its other lings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Disclaimer
Dr. Geaney may report consulting and royalty fees from Paragon 28 in connection with the provision of product development services to Paragon 28.
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.
References
-
“Population Clock,
USA Population”. United States Census Bureau, www.census.gov. Accessed 9 Sept. 2024. - Vanderkarr, Mari F., et al. "Incidence, costs and post-operative complications following ankle fracture–A US claims database analysis." BMC Musculoskeletal Disorders 23.1 (2022): 1129.
- Cancienne, Jourdan M., and Seth Yarboro. "Center-center syndesmosis fixation technique." Techniques in Foot & Ankle Surgery 14.3 (2015): 134-138.
- Wolfson, Theodore S., and Steven Struhl. "Continuous Loop Double Cortical Button Technique for Distal Tibiofibular Syndesmosis Stabilization: A Technical Note and Case Series." Techniques in Foot & Ankle Surgery 19.2 (2020): 104-113.
- Herzog, Mackenzie M., et al. "Epidemiology of ankle sprains and chronic ankle instability." Journal of athletic training 54.6 (2019): 603-610.
- Lehtonen, Eva J., et al. "Syndesmotic fixation with suture button: neurovascular structures at risk: a cadaver study." Foot & Ankle Specialist 13.1 (2020): 12-17.
- Peterson, Kyle S., et al. "Maintenance of reduction with suture button fixation devices for ankle syndesmosis repair." Foot & Ankle International 36.6 (2015): 679-684.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240904839497/en/
Investor Contact
Matthew Brinckman
Senior Vice President, Strategy and Investor Relations
Phone: (720) 912-1332
Source: Paragon 28, Inc.








