Paragon 28 Launches PRECISION® MIS Bunion System Offering Surgeons a Guided Minimally Invasive Surgical Solution for Treating Patients with Bunions
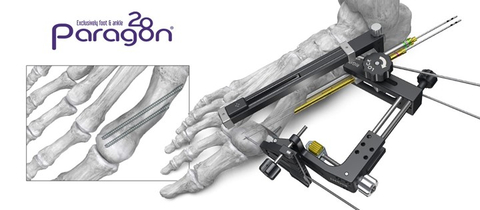
Figure 1: PRECISION® MIS Bunion System (Graphic: Business Wire)
The system features an outrigger designed for controlled tri-planar correction including translation and derotation of the metatarsal head and adjustment of the distal metatarsal articular angle. Instrumentation is also provided to facilitate a free-hand technique. The PRECISION® MIS Bunion System includes cannulated chamfer screws for fixation that allow alignment of the metatarsal head near the cortex of the first metatarsal. This minimally invasive technique is joint preserving, and screw placement is designed to reduce the chance of soft tissue irritation.
Surgeon designer David Gordon, MD, commented, “The PRECISION® MIS Bunion System by Paragon 28 really changes the landscape of minimally invasive hallux valgus correction, using a distal osteotomy. The design integrates all the elements essential for three-dimensional correction, including rotation. As the jig is fixed both distally and proximally, this gives it stability and the unique ability to apply compression across the osteotomy, to enhance healing. This jig has a high level of functionality and I am excited to see another thoughtful launch coming from Paragon 28.”
Paragon 28’s CEO, Albert DaCosta commented, “At Paragon 28, we have always been focused on matching solutions to the needs of the patient, and each patient is unique. We are adding a great new option to our suite of Bunion solutions with the launch of our PRECISION® MIS System that will help Paragon 28 and surgeons treat a broader array of patients with Hallux Valgus. I am proud of what our design surgeons and engineering team achieved in developing this system, which will bring benefits associated with minimally invasive surgery to our patients and surgeons in a reproducible way.”
Paragon 28 will debut the system at the American College of Foot and Ankle Surgeons (ACFAS) Annual Meeting this week in
The PRECISION® MIS Bunion System bolsters Paragon 28’s hallux valgus solutions offering which includes the Phantom® Small Bone Intramedullary Nail System, Gorilla® Lapidus Plating System, PRESERVE® Lapidus Wedge, Gorilla® MTP Plating System, PRESERVE™ MTP Wedge, PROMO™ Plating System, Bun-Yo-Matic™ Lapidus Clamp, Mini-Monster® Screw System and JAWS™ Staple System. With this comprehensive portfolio, Paragon 28® provides its customers with a single source to address their bunion needs.
About Paragon 28, Inc.
Based in
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward-looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward-looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and its Quarterly Reports on Form 10-Q, as updated periodically with its other filings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Disclaimer
Dr. Gordon may report consulting and royalty fees from Paragon 28 in connection with the provision of product development services to Paragon 28.
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240201226008/en/
Investor Contact
Matthew Brinckman
Senior Vice President, Strategy and Investor Relations
Phone: (720) 912-1332
mbrinckman@paragon28.com
Source: Paragon 28, Inc.







