Paragon 28 Launches JAWS™ Great White Staple System Further Expanding its Nitinol Staple Offering
- The JAWS™ Great White Staple System offers increased strength and stability for osteotomy or fusion sites, with an ultra-low-profile bridge and enhanced surface area to minimize soft tissue irritation. The staples provide 400 times the fatigue life of a competitive nitinol staple and a 169% increased compressive force compared to the average of a competitive marketing leading two-prong, nitinol staple. Paragon 28’s CEO, Albert DaCosta, expressed excitement about expanding their staple offering to address midfoot and hindfoot reconstruction complexities, enhancing market position in these segments.
- None.
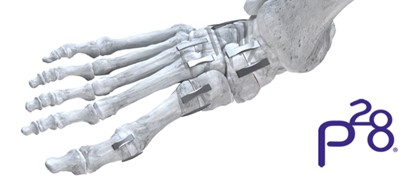
JAWS Great White Staple System (including 12, 15-, 18-, 20-, and 25-mm Staples) (Photo: Business Wire)
Paragon 28’s CEO, Albert DaCosta, commented, “We are excited to continue to expand our JAWS™ staple offering allowing surgeons to address the complexities of midfoot and hindfoot reconstruction. Our development team has designed implants which provide for greater fatigue resistance and increased compression when compared to market leading nitinol staple systems. We look forward to increasing our market position in the midfoot and hindfoot segments with this exciting offering.”
The addition of the JAWS™ Great White Staple System bolsters Paragon 28’s hindfoot solutions product offering, which includes the Gorilla® Ankle Fracture Plating System, APEX 3D™ Total Ankle Replacement, Silverback™ Ankle Fusion Plating System, Phantom® TTC Nail System, and Phantom® ActivCore™ Nail System. With this comprehensive portfolio, Paragon 28® provides its customers with innovative ankle solutions for trauma, arthritis, and limb salvage.
About Paragon 28, Inc.
Based in
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward‐looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward‐looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward‐looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and its Quarterly Reports on Form 10-Q, as updated periodically with its other filings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Disclaimer
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.
1. Internal Test Data on File
View source version on businesswire.com: https://www.businesswire.com/news/home/20231114327913/en/
Investor Contact
Matthew Brinckman
Senior Vice President, Strategy and Investor Relations
Phone: (720) 912-1332
mbrinckman@paragon28.com
Source: Paragon 28, Inc.
FAQ
What is the purpose of the JAWS™ Great White Staple System?
Who is the CEO of Paragon 28?







