Paragon 28 Launches Comprehensive Supramalleolar Osteotomy System
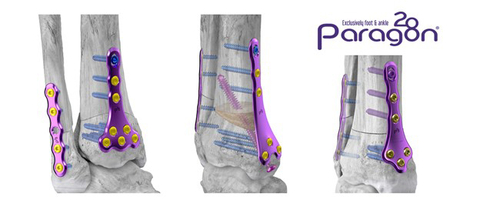
Gorilla® SMO Anterior Dome, Medial Closing Wedge, and Medial Opening Wedge Plating Configurations (Photo: Business Wire)
Mark Myerson, MD, Gorilla® SMO design surgeon, said, "Paragon28 has introduced an outstanding and precise supramalleolar osteotomy plating system. It is extremely accurate, enabling the surgeon to obtain optimal correction with cutting and drill guides and a truly unique and ingenious way to cut a PRESERVE™ Allograft Wedge for an opening wedge osteotomy."
Paragon 28’s CEO, Albert DaCosta, commented, “Hindfoot deformity correction and arthroplasty are two of the fastest growing segments in the foot and ankle market and the Gorilla® SMO Plating and Allograft System offers surgeons a streamlined and reproducible means to achieve deformity correction while preserving the ankle joint. We are very excited to have a comprehensive system which will allow surgeons to address tibial deformities and stage for total ankle arthroplasty supporting our growing APEX™ Total Ankle Arthroplasty System.”
The addition of the Gorilla® SMO Plating and Allograft System bolsters Paragon 28’s Precision Ankle Solutions product offering, which includes the Gorilla® Ankle Fracture Plating System, APEX 3D™ Total Ankle Replacement, Silverback™ Ankle Fusion Plating System, Phantom® TTC Nail System, and Phantom® ActivCore™ Nail System. With this comprehensive portfolio, Paragon 28® provides its customers with innovative ankle solutions for trauma, arthritis, and limb salvage.
Paragon 28® is grateful for the significant contributions that Woo Chun Lee, MD, Mark Myerson, MD, Cassandra Tomczak, DPM, and Federico Usuelli, MD made in the design of this system.
About Paragon 28, Inc.
Based in
View source version on businesswire.com: https://www.businesswire.com/news/home/20230523005148/en/
Investor Contact:
Matt Brinckman
Senior Vice President, Strategy and Investor Relations
(720) 912-1332
mbrinckman@paragon28.com
Source: Paragon 28, Inc.







