Paragon 28 Announces First Cases and Limited Market Release of the Bun-Yo-Matic™ – A Next Generation Clamp and Cut Guide System for Lapidus Bunion Procedures
- None.
- None.
Insights
The introduction of Paragon 28's Bun-Yo-Matic™ Lapidus Clamp System signifies a notable advancement in the realm of orthopedic surgery, specifically addressing bunion correction procedures. The device's ability to simulate weight-bearing and allow for compression prior to hardware application could potentially streamline the surgical process and enhance patient outcomes. This innovation is likely to be of interest to investors, as it may lead to increased adoption among surgeons and, consequently, potentially drive revenue growth for Paragon 28.
From a financial perspective, the recurring use of the clamp in surgeries could mean a stable stream of income from the sale of complementary implants and systems. This could be a strategic move to strengthen customer loyalty and increase market share in the competitive landscape of orthopedic devices. However, the level of market penetration and adoption rates by healthcare professionals will be crucial in determining the actual financial impact on the company's bottom line.
The Bun-Yo-Matic™ system represents a significant innovation in the technique of Lapidus arthrodesis, a procedure commonly performed to correct hallux valgus, or bunions. The ability to maintain anatomic alignment throughout the procedure could indeed lead to more consistent and optimal surgical outcomes. The integration of the device with existing Paragon 28 products may also facilitate ease of use for surgeons already familiar with the company's portfolio.
It is important to note that while the system promises improved accuracy and reproducibility, the actual benefit to patient outcomes will need to be validated through clinical studies and post-market surveillance. Nevertheless, the emphasis on procedural efficiency and potential reduction in operative time could resonate well with surgical teams, potentially leading to increased adoption of the system.
The limited market release of the Bun-Yo-Matic™ system by Paragon 28 could be interpreted as a strategic move to test market response before a wider rollout. The feedback and data collected from the initial surgical cases in Orange County, California and Seattle, Washington, will be critical in refining the product and marketing strategy. The emphasis on reproducibility and efficiency aligns with current healthcare trends that prioritize value-based care and patient outcomes.
Market research will need to focus on the acceptance of this new technology by orthopedic surgeons and the potential impact on the competitive landscape. The bunion correction market is a niche but significant segment of orthopedic devices and innovations that offer tangible benefits are well-positioned to capture market share. The long-term success of the Bun-Yo-Matic™ system will depend on its clinical performance and the company's ability to effectively communicate its value proposition to both surgeons and healthcare institutions.
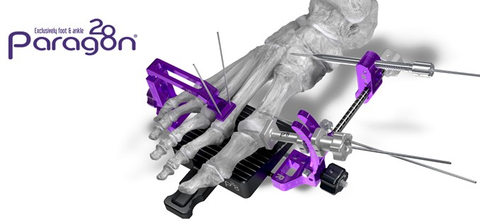
Figure 1: Paragon 28 Bun-Yo-Matic™ Lapidus Clamp System (Graphic: Paragon 28)
The Bun-Yo-Matic™ Clamp remains fixed to the anatomy throughout the entire procedure, ensuring surgeon-guided correction and anatomic alignment are maintained through application of fixation. The System is also equipped with cutting guides designed to minimize bone removal from the base of Tarsometatarsal (“TMT”) joint when preparing for fusion.
The Bun-Yo-Matic™ Clamp and accompanying cut guide system are highly complementary to Paragon 28’s broad line of bunion and forefoot solutions. The Clamp is a reusable surgical instrument which will be used in conjunction with several of Paragon 28’s existing implants and implant systems designed to treat bunions, including:
- Gorilla® Precision™ Lapidus Plate
- Phantom® Small Bone Intramedullary Nail
- Phantom® MIS Lapidus System allowing medial access
- MIS Burr Guide used in preparing the 1st TMT joint
Paragon 28’s CEO, Albert DaCosta, commented, “The first clinical cases with the Bun-Yo-Matic™ Clamp System went great, and we expect this product to drive significant growth for Paragon 28. We believe this clamp is a game changer in the bunion market and will result in more reproducibility, streamlined procedural work flow, improved accuracy, and ultimately improved patient outcomes in technically demanding Lapidus arthrodesis cases. With the capability to hold correction across all three planes throughout the entire procedure, the system is unique and brings great value to our surgeons and patients.”
The Bun-Yo-matic™ Lapidus Clamp System bolsters Paragon 28’s bunion and forefoot solutions offering which includes the Gorilla® Precision™ Lapidus Plate, Phantom® Small Bone Intramedullary Nail, Phantom® MIS System, Gorilla® Precision MTP Plate, Phantom® Metatarsal Shortening System, TenoTac™, Paratrooper™, and HammerTube™. With this comprehensive portfolio, Paragon 28® provides its customers with a single source to address their bunion and forefoot needs.
About Paragon 28, Inc.
Based in
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward‐looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward‐looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward‐looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and its Quarterly Reports on Form 10-Q, as updated periodically with its other filings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Disclaimer
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240117544407/en/
Investor Contact
Matthew Brinckman
Senior Vice President, Strategy and Investor Relations
Phone: (720) 912-1332
Source: Paragon 28, Inc.







