Paragon 28 Advances Foot and Ankle Specific Soft Tissue Portfolio with Launch of Grappler® Knotless Anchor System and Innovative Bridgeline™ Tape
- None.
- None.
Insights
The introduction of the Grappler® Knotless Anchor System and Bridgeline™ Tape by Paragon 28 represents a strategic expansion within the foot and ankle soft tissue repair market. This segment has seen increasing demand due to a growing number of sports injuries and an aging population susceptible to musculoskeletal disorders. The company's emphasis on innovation and surgeon-centric design is likely to enhance its competitive edge in this specialized field.
From a product standpoint, the integration of these new offerings with Paragon 28's existing hardware portfolio could streamline surgical procedures and reduce operational complexity, potentially leading to cost savings for healthcare providers. Furthermore, the semi-resorbable nature of the Bridgeline™ Tape, which aligns with the body's healing process, suggests a potential for improved patient outcomes and reduced long-term healthcare costs.
For investors, the launch of these products may indicate sustainable growth potential for Paragon 28. The company's focus on product differentiation and the ability to address a comprehensive range of soft tissue injuries could result in increased market share and strengthen its position in the orthopedic device sector.
The market for foot and ankle orthopedic devices is projected to grow significantly, driven by technological advancements and an increase in orthopedic procedures. Paragon 28's launch of the Grappler® Knotless Anchor System and Bridgeline™ Tape aligns with industry trends favoring minimally invasive surgeries and products that promote faster recovery times.
In terms of market dynamics, Paragon 28's strategy to offer a full suite of products for foot and ankle soft tissue repair could enhance its bargaining power with hospitals and clinics, enabling bundle pricing strategies and fostering customer loyalty. The company's product innovation may also stimulate demand from orthopedic surgeons looking for state-of-the-art surgical aids.
Analyzing the potential market impact, if these products deliver on their promise of improved surgical outcomes, they could rapidly gain adoption, translating into increased revenue streams for Paragon 28. However, the company must navigate stringent regulatory landscapes and demonstrate the clinical efficacy of their products to capitalize on these opportunities.
The Grappler® Knotless Anchor System and Bridgeline™ Tape represent significant advancements in the field of foot and ankle surgery. The ergonomics and design of the Grappler® Suture Anchor System, tailored for foot and ankle applications, could offer surgeons increased precision and ease during procedures, which is crucial for successful patient outcomes.
The semi-resorbable characteristic of the Bridgeline™ Tape is particularly noteworthy. The ability of a stabilization material to adapt over time from a rigid state to one that more closely mimics the natural physiological behavior of soft tissue could potentially reduce the incidence of complications such as scarring or re-injury.
From a clinical perspective, the integration of these systems into Paragon 28's portfolio may facilitate more efficient surgical workflows and improve the standard of care for patients with soft tissue injuries. Surgeons may experience a learning curve with the adoption of these new technologies, but the long-term benefits could be substantial both for patient recovery and for reducing the overall burden on healthcare systems.
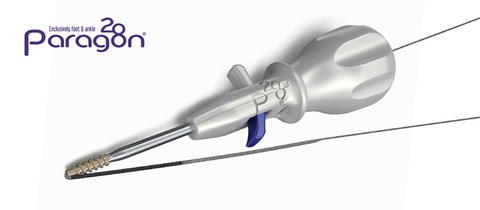
Figure 1: Grappler® Knotless Anchor System and Bridgeline™ Tape (Photo: Business Wire)
The Grappler® Suture Anchor System is a sterile packed kit that includes all instrumentation needed to complete each case in multiple configurations designed to reduce waste and interoperative complexity.
Surgeon designer, Mark Prissel, DPM commented, “The Grappler® Suture Anchor System is a comprehensive soft tissue anchoring product line that includes implants designed by and for foot and ankle surgeons for acute and chronic repairs. The implant sizes, suture lengths, and instrument ergonomics are all ideally equipped for efficient utilization in common tendon and ligament procedures, while complementing the broader Paragon 28 product portfolio seamlessly.”
The Bridgeline™ Tape is a huge leap forward in soft tissue stabilization. The tape features a novel semi-resorbable material with the ability to transition from rigid at the early stages of healing to a more natural physiological state. This product’s ability to transition over time is truly unique and is believed to foster better healing characteristics and expected to result in improved patient outcomes.
“We continue to believe soft tissue will play an increasingly important role in improving foot and ankle patient outcomes, and we could not be more excited to bring two new solutions to market to start the year. Many of the procedures we aim to treat at Paragon 28 involve some element of soft tissue compromise and we expect both of these products to be complementary to a large portion of our portfolio,” commented Albert DaCosta, Paragon 28’s CEO. “I am especially proud of what we accomplished with the Bridgeline™ Tape. With that product, we set out with a desire to create an optimal environment for healing with less scarring and we believe this unique solution accomplishes that goal and, importantly, will result in optimal treatment for each patient.”
The Grappler® Knotless Anchor System and Bridgeline™ Tape bolsters Paragon 28’s soft tissue offering which includes the Paratrooper™ Plantar Plate System, TenoTac™ 2.0 Soft Tissue Fixation System, Grappler® Interference Screw System, and Grappler Soft Tissue Anchor System. With this comprehensive portfolio, Paragon 28® provides its customers with a single source to address their foot and ankle soft tissue needs.
About Paragon 28, Inc.
Based in
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward‐looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward‐looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward‐looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and its Quarterly Reports on Form 10-Q, as updated periodically with its other filings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Disclaimer
Dr. Prissel may report consulting and royalty fees from Paragon 28 in connection with the provision of product development services to Paragon 28.
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240105897232/en/
Investor Contact
Matthew Brinckman
Senior Vice President, Strategy and Investor Relations
Phone: (720) 912-1332
Source: Paragon 28, Inc.
FAQ
What did Paragon 28, Inc. announce?
What is the ticker symbol for Paragon 28, Inc.?
What are the features of the Grappler® Knotless Anchor System?
What is the Bridgeline™ Tape?
Who commented on the Grappler® Suture Anchor System?







