Paragon 28 Adds to Suite of Novel Syndesmosis Repair Solutions with Launch of Grappler® R3INFORCE™ Extraosseous Repair System
- None.
- None.
Insights
The introduction of Paragon 28's Grappler® R3INFORCE™ Extraosseous Repair System represents a significant advancement in the field of orthopedic medical devices, specifically those used in the treatment of ankle injuries. The system's focus on restoring stability to ligaments surrounding the ankle addresses a clear clinical need, as a considerable proportion of ankle fractures and high ankle sprains require surgical intervention for proper healing.
From a market perspective, the compatibility of the new system with existing Grappler® products may streamline surgical procedures and reduce the need for multiple devices, which could potentially lead to cost savings for healthcare providers and improved outcomes for patients. The emphasis on reducing waste and intraoperative complexity could also enhance operating room efficiency, a factor that is increasingly valued in the healthcare industry. Moreover, the sterile packaging and inclusion of necessary instrumentation further simplify the surgical process, potentially leading to quicker adoption rates among surgeons.
Considering the global orthopedic devices market, innovations like the Grappler® R3INFORCE™ System may contribute to the company's market share growth. However, it is essential to monitor the system's clinical outcomes and adoption rate post-launch to gauge its impact on the company's financial performance.
The Grappler® R3INFORCE™ Extraosseous Repair System appears to offer a unique approach to the treatment of syndesmotic injuries, which are often challenging to manage due to the complex anatomy and biomechanics of the ankle. The Dynamic Anchor's allowance for micromotion could potentially provide a more physiological repair by mimicking the natural movement of the ankle, which may improve patient outcomes.
The system's design for multiple configurations offers surgeons the flexibility to tailor the repair to individual patient needs, which is a critical aspect of personalized medicine. While the system's benefits are clear, long-term studies would be necessary to validate its efficacy compared to traditional methods of syndesmotic stabilization.
As an orthopedic surgeon, it's exciting to see innovation that could enhance the standard of care for ankle injuries, but the true test will be in the clinical results and the system's ability to facilitate quicker, more effective patient recoveries.
Paragon 28's launch of the Grappler® R3INFORCE™ system could potentially capture a significant niche in the orthopedic device segment. The focus on addressing up to 25% of all ankle fractures and unstable high ankle sprains presents a targeted approach to a common medical issue. The company's strategy to build a comprehensive suite of products for foot and ankle soft tissue injuries aligns with current healthcare trends towards specialization and complete solutions.
However, the success of the product will largely depend on its clinical adoption. Factors such as ease of use, cost-effectiveness and integration into existing surgical workflows will be critical in determining its market penetration. Additionally, the company's ability to effectively communicate the benefits of the system to both surgeons and hospital procurement departments will be essential.
The long-term impact on Paragon 28's business will be contingent upon the system's ability to displace existing solutions and become a preferred method for syndesmotic injury repair. Market reception, surgeon training and patient outcomes will be key indicators to watch in the coming quarters.
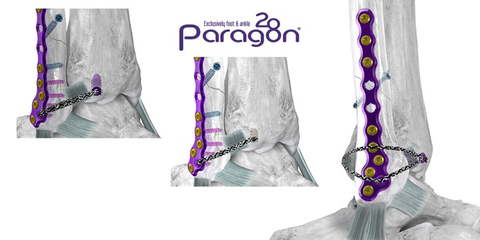
Figure 1: Anterior inferior tibiofibular ligament (“AITFL”) Repair Showing the Grappler® R3INFORCE™ Dynamic Anchor (left) and Grappler® Knotless Anchor (center). Complete Extraosseous Repair with Grappler® R3INFORCE™ System shown on right. (Graphic: Business Wire)
Upon determination of syndesmotic instability, the Grappler® R3INFORCE™ Anchors are placed in plate holes or other anatomic locations where pre-loaded suture tape can be routed and tensioned to address the injured ligament or ligaments. The Dynamic Anchor allows for micromotion in the repair to better replicate the mechanics of the native tissue.
This system is highly synergistic with Paragon 28’s existing offerings and can utilize the recently launched Grappler® Knotless Sutures. This compatibility allows for multiple configurations for surgeons to cater their syndesmotic repair to their own treatment philosophies.
The Grappler® R3INFORCE™ Extraosseous Repair System is sterile packed and includes all instrumentation needed to complete the case in multiple configurations, reducing waste and intraoperative complexity.
Paragon 28’s CEO, Albert DaCosta, commented, “With up to
Surgeon Designer Mark Davies, MD, commented, “Congratulations to the blue sky thinking and creativity of the Paragon 28 team in manufacturing the Grappler® R3INFORCE™ Extraosseous Repair System. It offers surgeons a comprehensive, physiological, and anatomical reconstruction system for ankle syndesmotic injuries.”
The Grappler® R3INFORCE™ Extraosseous Repair System bolsters Paragon 28’s soft tissue offering, which includes the Paratrooper™ Plantar Plate System, TenoTac™ 2.0 Soft Tissue Fixation System, Grappler® Interference Screw System, Grappler® Suture Anchor System, Grappler® Knotless Anchor System, Bridgeline™ Tape, R3ACT® Stabilization System, R3LEASE™ Stabilization System, and Mister Tendon™ Harvester. With this comprehensive portfolio, Paragon 28® provides its customers with a single source to address their foot and ankle soft tissue needs.
About Paragon 28, Inc.
Based in
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward‐looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward‐looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward‐looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and its Quarterly Reports on Form 10-Q, as updated periodically with its other filings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Disclaimer
Dr. Davies may report consulting and royalty fees from Paragon 28 in connection with the provision of product development services to Paragon 28.
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240328312304/en/
Investor Contact
Matthew Brinckman
Senior Vice President, Strategy and Investor Relations
Phone: (720) 912-1332
Source: Paragon 28, Inc.







