Eyenovia Announces Progress on Next-Generation User-Filled Optejet Dispensing Device
Eyenovia (NASDAQ: EYEN) announced progress on its user-filled Optejet® spray dispenser, planning U.S. regulatory submission in Q4 2025. The device aims to address common issues with traditional eye drops, including inaccurate administration and discomfort.
Key features of the next-generation Optejet include: a sterile disposable cartridge that users can fill from their eyedropper bottle, capable of 180 metered sprays; demonstrated durability with the base unit performing over 30,000 sprays; and 98% spray accuracy between 8-9 microliters. The device is designed for various topical ophthalmic liquids, targeting the artificial tears and lens rewetting market, expected to reach $4 billion in U.S. sales this year.
Eyenovia (NASDAQ: EYEN) ha annunciato progressi nel suo dispenser di spray Optejet® riempito dagli utenti, pianificando la presentazione della domanda regolatoria negli Stati Uniti nel quarto trimestre del 2025. Il dispositivo mira a risolvere problemi comuni con le gocce oculari tradizionali, tra cui la somministrazione imprecisa e il disagio.
Le principali caratteristiche del nuovo Optejet di prossima generazione includono: un caricatore sterile monouso che gli utenti possono riempire dalla loro bottiglia di gocce oculari, capace di 180 spruzzi misurati; durevolezza dimostrata con l'unità base che esegue oltre 30.000 spruzzi; e un'accuratezza di spruzzo del 98% tra 8-9 microlitri. Il dispositivo è progettato per vari liquidi oftalmici topici, puntando al mercato delle lacrime artificiali e del reidratante per lenti, che si prevede raggiungerà 4 miliardi di dollari in vendite negli Stati Uniti quest'anno.
Eyenovia (NASDAQ: EYEN) anunció avances en su dispensador de aerosol Optejet® rellenado por los usuarios, con planes para presentar la solicitud regulatoria en EE. UU. en el cuarto trimestre de 2025. El dispositivo tiene como objetivo abordar problemas comunes con las gotas para los ojos tradicionales, incluyendo la administración inexacta y la incomodidad.
Las características clave del Optejet de nueva generación incluyen: un cartucho desechable estéril que los usuarios pueden llenar desde su botella de gotas para los ojos, capaz de 180 pulverizaciones medidas; durabilidad demostrada con la unidad base que realiza más de 30,000 pulverizaciones; y una precisión de pulverización del 98% entre 8-9 microlitros. El dispositivo está diseñado para varios líquidos oftálmicos tópicos, con enfoque en el mercado de lágrimas artificiales y humectantes para lentes, que se espera alcance los $4 mil millones en ventas en EE. UU. este año.
아이노비아 (NASDAQ: EYEN)가 사용자 채우기식 오프테젯® 스프레이 분배기의 진행 상황을 발표하며, 2025년 4분기 미국 규제 제출을 계획하고 있습니다. 이 장치는 전통적인 안약과 관련된 일반적인 문제인 부정확한 투여와 불편함을 해결하는 것을 목표로 하고 있습니다.
차세대 오프테젯의 주요 기능으로는: 사용자가 자신의 안약 병에서 채울 수 있는 일회용 무균 카트리지로 180회의 측정된 스프레이가 가능하며; 기본 장치가 30,000회 이상의 스프레이를 수행할 수 있는 내구성이 입증되었으며; 8-9 마이크롤리터에서 98%의 스프레이 정확도를 보여줍니다. 이 장치는 다양한 국소 안과 액체를 대상으로 설계되었으며, 인공 눈물과 렌즈 재습윤 시장을 겨냥하고 있으며, 올해 미국에서 40억 달러의 판매에 이를 것으로 예상됩니다.
Eyenovia (NASDAQ: EYEN) a annoncé des progrès concernant son distributeur de spray Optejet® rempli par les utilisateurs, avec une soumission réglementaire prévue aux États-Unis au quatrième trimestre 2025. L'appareil vise à résoudre des problèmes courants liés aux gouttes ophtalmiques traditionnelles, notamment l'administration inexacte et l'inconfort.
Parmi les principales caractéristiques de la prochaine génération d'Optejet figurent : une cartridge jetable stérile que les utilisateurs peuvent remplir depuis leur flacon de gouttes oculaires, capable de 180 pulvérisations dosées ; une durabilité démontrée avec l'unité de base effectuant plus de 30 000 pulvérisations ; et une précision de pulvérisation de 98 % entre 8 et 9 microlitres. L'appareil est conçu pour divers liquides ophtalmiques topiques, visant le marché des larmes artificielles et de l'humidification des lentilles, qui devrait atteindre 4 milliards de dollars de ventes aux États-Unis cette année.
Eyenovia (NASDAQ: EYEN) hat Fortschritte bei seinem benutzergefüllten Optejet®-Sprühspender angekündigt und plant die Einreichung bei den US-Behörden im vierten Quartal 2025. Das Gerät soll gängige Probleme mit herkömmlichen Augentropfen, einschließlich ungenauer Anwendung und Unbehagen, beheben.
Zu den wichtigsten Funktionen des nächsten Optejet-Generationsmodells gehören: eine sterile Einwegkartusche, die Benutzer aus ihrer Augentropfenflasche füllen können, mit der Möglichkeit von 180 dosierten Sprühstößen; demonstrierte Haltbarkeit mit der Basiseinheit, die über 30.000 Sprühstöße durchführt; und eine Sprühgenauigkeit von 98% bei 8-9 Mikrolitern. Das Gerät ist für verschiedene topische ophthalmologische Flüssigkeiten ausgelegt, mit einem Fokus auf den Markt für künstliche Tränen und Kontaktlinsen-Befeuchtung, der in diesem Jahr voraussichtlich einen Umsatz von 4 Milliarden Dollar in den USA erreichen wird.
- Device demonstrates 98% accuracy in spray delivery
- Base unit shows durability with over 30,000 spray capability
- Targets $4 billion U.S. market for artificial tears and lens rewetting products
- Regulatory submission planned for Q4 2025
- Product still pending regulatory approval
- Commercialization timeline uncertain
Insights
Eyenovia's progress with their next-generation Optejet device marks a pivotal advancement in ophthalmic drug delivery technology. The device's 98% spray consistency between 8-9 microliters is particularly noteworthy, as this precise dosing addresses a significant limitation of traditional eye drops, which typically deliver 30-50 microliters, resulting in significant waste and potential side effects.
The user-filled cartridge design represents a clever market entry strategy. By allowing compatibility with existing eye drop products, Eyenovia sidesteps the need for extensive drug development and can potentially capture market share across multiple existing product categories. The 30,000-spray durability of the base unit suggests robust engineering that could support a premium pricing strategy while maintaining long-term value for users.
The planned Q4 regulatory submission timing is strategic, as it positions Eyenovia to potentially enter the market in 2026, when the artificial tears and lens rewetting market is expected to continue growing beyond its current
Key technical advantages include:
- Reduced waste through precise microspray technology
- Enhanced user experience with simplified administration
- Potential cost savings through more efficient drug utilization
- Cross-compatibility with existing eye drop formulations
Eyenovia plans to submit for U.S. device regulatory approval in Q4 of this year, marking a key step toward commercialization
NEW YORK, Feb. 05, 2025 (GLOBE NEWSWIRE) -- Eyenovia, Inc. (NASDAQ: EYEN) (“Eyenovia” or the “Company”), an ophthalmic technology company focused on completing development of its proprietary Optejet® device, today announces recent progress on the development of its user-filled spray dispenser.
“Millions of consumers have difficulty with traditional eye drops, including difficulty with inaccurate administration, discomfort from head tilting, messing up make-up and waste and potential side effects associated with excess drops, all of which could be addressed with the Optejet,” stated Michael Rowe, Chief Executive Officer of Eyenovia. “To that end, we continue to advance development of our novel, user-filled Optejet dispenser and look forward to filing for U.S. regulatory approval for this device in the fourth quarter of this year. We believe the user-filled Optejet can address multi-billion-dollar ophthalmic markets while offering an enhanced user experience.”
Some of the features incorporated into the user-filled Optejet include:
User-Filled Cartridge
The new design includes a sterile disposable cartridge that users can fill using their own, fresh eyedropper bottle. The cartridge is then attached to the reusable base unit and would be capable of dispensing up to 180 metered sprays. Once empty, the cartridge is simply replaced with a new user-filled cartridge.
Reliable and Precise Spray
Rigorous testing has resulted in observations of the Optejet’s durable base unit performing over 30,000 sprays, and
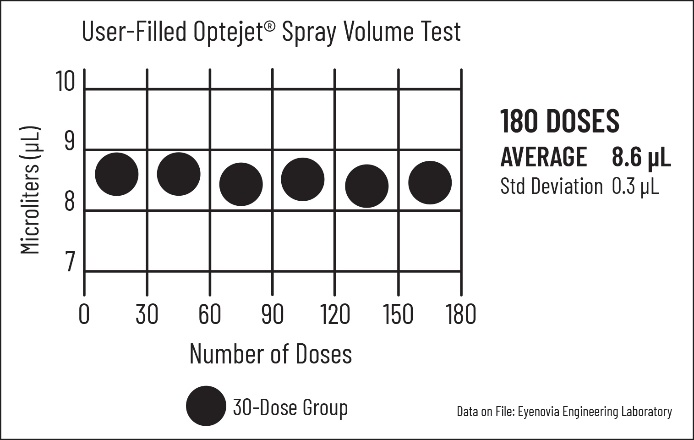
Versatile Applications
The user-filled Optejet is designed to work with a variety of topical ophthalmic liquids, such as artificial tears and lens rewetting products, which are expected to generate sales of four billion dollars in the U.S. this year alone.
About Eyenovia, Inc.
Eyenovia, Inc. is an ophthalmic technology company developing its proprietary Optejet topical ophthalmic medication dispensing platform. The Optejet may be especially useful in treatment of chronic front-of-the-eye diseases due to its ease of use, enhanced safety and tolerability, and potential for superior compliance versus standard eye drops. Together, these benefits may combine to produce better treatment options and outcomes for patients and providers. For more information, please visit Eyenovia.com.
Forward Looking Statements
Except for historical information, all the statements, expectations and assumptions contained in this press release are forward-looking statements. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions, including those relating to the estimated market opportunities for our platform technology and the regulatory pathway and timing for availability of our products. These statements are based on current expectations, estimates and projections about our business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may, and in some cases are likely to, differ materially from what is expressed or forecasted in the forward-looking statements due to numerous factors discussed from time to time in documents which we file with the U.S. Securities and Exchange Commission.
In addition, such statements could be affected by risks and uncertainties related to, among other things: the potential advantages of our products and platform technology; the regulatory pathway that would apply to our products; our estimates regarding the potential market opportunity for our products; reliance on third parties to develop and commercialize our products; the ability of us and our partners to timely develop, implement and maintain manufacturing, commercialization and marketing capabilities and strategies for our products; intellectual property risks; changes in legal, regulatory, legislative and geopolitical environments in the markets in which we operate and the impact of these changes on our ability to obtain and maintain regulatory approval for our products and product candidates; our competitive position; our ability to raise additional funds and to make payments on our debt obligations as and when necessary; and our ability to pursue strategic alternatives.
Any forward-looking statements speak only as of the date on which they are made, and except as may be required under applicable securities laws, Eyenovia does not undertake any obligation to update any forward-looking statements.
Eyenovia Contact:
Eyenovia, Inc.
Norbert Lowe
Sr. Vice President, Commercial Operations
admin@eyenovia.com
Eyenovia Investor Contact:
Eric Ribner
LifeSci Advisors, LLC
eric@lifesciadvisors.com
(646) 751-4363
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/405b239a-8b0f-4d1f-a650-aa6bf45d84e9








