Edgewise Therapeutics Announces Positive Top-Line Data from Phase 1 Trial in Healthy Subjects and Phase 2 CIRRUS-HCM Trial in Patients with Obstructive Hypertrophic Cardiomyopathy (HCM)
Edgewise Therapeutics (Nasdaq: EWTX) announced positive top-line data for EDG-7500, a novel oral cardiac sarcomere modulator, from its Phase 1 trial in healthy subjects and Phase 2 CIRRUS-HCM trial in patients with obstructive hypertrophic cardiomyopathy (HCM). Key findings include:
1. Phase 1: EDG-7500 was well-tolerated without meaningful changes in left ventricle ejection fraction (LVEF).
2. CIRRUS-HCM: Single-dose trial demonstrated robust left ventricular outflow tract (LVOT) gradient reductions without significant LVEF changes.
3. 67% mean reduction in resting LVOT pressure gradient and 55% mean reduction in provokable LVOT gradient observed at 100 and 200 mg doses.
4. 64% mean reduction in NT-proBNP, a key heart failure biomarker, in the 200 mg cohort.
The company has initiated the 28-day part of CIRRUS-HCM in patients with obstructive and non-obstructive HCM, with initial data expected in Q1 2025.
Edgewise Therapeutics (Nasdaq: EWTX) ha annunciato dati positivi di fase per EDG-7500, un nuovo modulatoro cardiaco orale del sarcomero, provenienti dal suo studio di Fase 1 su soggetti sani e dallo studio di Fase 2 CIRRUS-HCM su pazienti con cardiomiopatia ipertrofica ostruttiva (HCM). I risultati chiave includono:
1. Fase 1: EDG-7500 ha mostrato una buona tollerabilità senza cambiamenti significativi della frazione di eiezione del ventricolo sinistro (LVEF).
2. CIRRUS-HCM: Lo studio con dose singola ha dimostrato riduzioni significative del gradiente di flusso ventricolare sinistro (LVOT) senza cambiamenti rilevanti della LVEF.
3. Riduzione media del 67% nel gradiente di pressione LVOT a riposo e riduzione media del 55% nel gradiente LVOT provocabile osservato a dosi di 100 e 200 mg.
4. Riduzione media del 64% nel NT-proBNP, un biomarcatore chiave per l'insufficienza cardiaca, nel gruppo da 200 mg.
La compagnia ha avviato la parte di 28 giorni di CIRRUS-HCM in pazienti con HCM ostruttiva e non ostruttiva, con dati iniziali attesi nel Q1 2025.
Edgewise Therapeutics (Nasdaq: EWTX) anunció datos positivos de fase para EDG-7500, un nuevo modulador oral del sarcómero cardíaco, provenientes de su ensayo de Fase 1 en sujetos sanos y del ensayo de Fase 2 CIRRUS-HCM en pacientes con miocardiopatía hipertrófica obstructiva (HCM). Los hallazgos clave incluyen:
1. Fase 1: EDG-7500 fue bien tolerado sin cambios significativos en la fracción de eyección del ventrículo izquierdo (LVEF).
2. CIRRUS-HCM: El ensayo de dosis única demostró reducciones sólidas en el gradiente de salida del ventrículo izquierdo (LVOT) sin cambios significativos en la LVEF.
3. Reducción media del 67% en el gradiente de presión LVOT en reposo y reducción media del 55% en el gradiente LVOT provocable observada a dosis de 100 y 200 mg.
4. Reducción media del 64% en NT-proBNP, un biomarcador clave para la insuficiencia cardíaca, en el grupo de 200 mg.
La compañía ha iniciado la parte de 28 días de CIRRUS-HCM en pacientes con HCM obstructiva y no obstructiva, con datos iniciales esperados para el primer trimestre de 2025.
엣지와이즈 테라퓨틱스(나스닥: EWTX)는 건강한 피험자들을 대상으로 한 1상 시험과 폐쇄성 비대 심근병증(HCM) 환자들을 대상으로 한 2상 CIRRUS-HCM 시험에서 새로운 경구 심장 사르코메어 조절제 EDG-7500의 긍정적인 초기 데이터를 발표했습니다. 주요 발견 사항은 다음과 같습니다:
1. 1상: EDG-7500은 좌심실 박출 분율(LVEF)의 의미 있는 변화 없이 잘 견딜 수 있는 것으로 나타났습니다.
2. CIRRUS-HCM: 단일 용량 시험은 LVEF의 중요한 변화 없이 강력한 좌심실 유출로(LVOT) 기울기 감소를 보여주었습니다.
3. 100mg 및 200mg 용량에서 안정 시 LVOT 압력 기울기의 평균 67% 감소와 유발 가능한 LVOT 기울기의 평균 55% 감소가 관찰되었습니다.
4. 200mg 집단에서 심부전의 주요 바이오마커인 NT-proBNP의 평균 64% 감소가 나타났습니다.
회사는 폐쇄성 및 비폐쇄성 HCM 환자들을 대상으로 CIRRUS-HCM의 28일 부분을 시작했으며, 초기 데이터는 2025년 1분기에 발표될 예정입니다.
Edgewise Therapeutics (Nasdaq: EWTX) a annoncé des données positives de première ligne pour EDG-7500, un nouveau modulateur oral de sarcomère cardiaque, provenant de son essai de phase 1 sur des sujets en bonne santé et de l'essai de phase 2 CIRRUS-HCM chez des patients souffrant de cardiomyopathie hypertrophique obstructive (HCM). Les résultats clés comprennent:
1. Phase 1: EDG-7500 a été bien toléré sans changements significatifs de la fraction d'éjection du ventricule gauche (LVEF).
2. CIRRUS-HCM: L'essai à dose unique a démontré des réductions importantes du gradient de circulation ventriculaire gauche (LVOT) sans changements significatifs de la LVEF.
3. Réduction moyenne de 67% du gradient de pression LVOT au repos et réduction moyenne de 55% du gradient LVOT provoqué observée à des doses de 100 et 200 mg.
4. Réduction moyenne de 64% du NT-proBNP, un biomarqueur clé de l'insuffisance cardiaque, dans le groupe de 200 mg.
L'entreprise a lancé la partie de 28 jours du CIRRUS-HCM chez des patients atteints de HCM obstructive et non obstructive, avec des données initiales prévues pour le 1er trimestre 2025.
Edgewise Therapeutics (Nasdaq: EWTX) hat positive Top-Line-Daten zu EDG-7500, einem neuartigen oralen kardialen Sarkomer-Modulator, aus seiner Phase-1-Studie mit gesunden Probanden und der Phase-2-CIRRUS-HCM-Studie bei Patienten mit obstruktiver hypertropher Kardiomyopathie (HCM) bekannt gegeben. Wichtige Ergebnisse sind:
1. Phase 1: EDG-7500 wurde gut vertragen, ohne bedeutende Veränderungen der linksventrikulären Auswurfquote (LVEF).
2. CIRRUS-HCM: Die Einzeldosis-Studie zeigte signifikante Reduktionen des gradienten im linken Ventrikelabfluss (LVOT) ohne signifikante Änderungen in der LVEF.
3. Durchschnittliche Reduktion von 67% beim Ruhe-LVOT-Druckgradienten und durchschnittliche Reduktion von 55% beim auslösbaren LVOT-Gradienten bei Dosen von 100 und 200 mg.
4. Durchschnittliche Reduktion von 64% im NT-proBNP, einem wichtigen Biomarker für Herzinsuffizienz, in der 200-mg-Kohorte.
Das Unternehmen hat den 28-tägigen Teil von CIRRUS-HCM bei Patienten mit obstruktiver und nicht-obstruktiver HCM gestartet, die ersten Daten werden im 1. Quartal 2025 erwartet.
- EDG-7500 demonstrated robust LVOT gradient reductions in HCM patients
- No meaningful changes in LVEF observed across a broad range of EDG-7500 exposures
- 64% mean reduction in NT-proBNP, a key heart failure biomarker, in the 200 mg cohort
- EDG-7500 was well-tolerated in both single and multiple ascending dose trials
- Initiation of 28-day CIRRUS-HCM trial in obstructive and non-obstructive HCM patients
- None.
Insights
The Phase 1 and Phase 2 CIRRUS-HCM trial results for EDG-7500 are highly promising for Edgewise Therapeutics. Key findings include:
- Well-tolerated in healthy subjects with no significant safety concerns
- 67% mean reduction in resting LVOT pressure gradient in HCM patients
- 55% mean reduction in provokable LVOT pressure gradient
- 64% mean reduction in NT-proBNP, a heart failure biomarker
- No meaningful changes in LVEF across a broad range of exposures
These results suggest EDG-7500 could potentially address both obstructive and non-obstructive HCM, offering a novel treatment option in an area of unmet medical need. The initiation of the 28-day CIRRUS-HCM trial further supports the compound's potential.
The data on EDG-7500 is particularly intriguing from a clinical perspective. The ability to significantly reduce LVOT gradients without compromising left ventricular function (LVEF) is a notable achievement. This suggests a potential for improved symptom management and possibly better long-term outcomes for HCM patients. The 60% of patients achieving LVOT gradients <30 mmHg at rest and <50 mmHg with Valsalva is clinically meaningful. The substantial reduction in NT-proBNP levels also hints at potential benefits beyond just obstructive HCM, possibly extending to non-obstructive HCM and other forms of diastolic dysfunction. However, longer-term data will be important to fully assess the drug's efficacy and safety profile.
From a financial perspective, these results are potentially game-changing for Edgewise Therapeutics (NASDAQ: EWTX). The positive data from both Phase 1 and Phase 2 trials significantly de-risks the EDG-7500 program, which could lead to increased investor confidence and potential partnerships or licensing deals. The addressable market for HCM treatments is substantial and if EDG-7500 continues to show efficacy in both obstructive and non-obstructive HCM, it could capture a significant market share. Investors should watch for the 28-day data expected in Q1 2025, as positive results could be a major catalyst for the stock. However, it's important to note that further clinical development and regulatory hurdles remain before potential commercialization.
– In a Phase 1 in healthy subjects, EDG-7500 was well-tolerated without meaningful changes in left ventricle ejection fraction (LVEF) –
– CIRRUS-HCM single-dose trial of EDG-7500 in obstructive HCM demonstrated robust left ventricular outflow tract (LVOT) gradient reductions without meaningful changes in LVEF –
– Company announced dosing of first patients in CIRRUS-HCM 28-day trial –
– Edgewise to host webcast event on Thursday, September 19 at 8:30 a.m. Eastern Time –
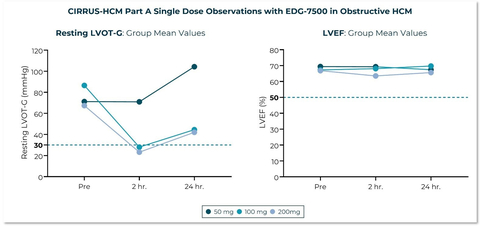
CIRRUS-HCM Part A Single Dose Observations with EDG-7500 in Obstructive HCM (Graphic: Business Wire)
In the placebo-controlled Phase 1 single ascending dose (SAD) trial (n=48), healthy subjects received single doses of EDG-7500, ranging from 5 to 300 mg. In the multiple ascending dose (MAD) portion of the trial (n=24), healthy subjects received 25 to 100 mg once daily for 14 days. EDG-7500 was well tolerated in both the SAD and MAD; there were no clinically meaningful changes or trends in vital signs, clinical chemistry, hematology, or electrocardiograms. There were no meaningful changes in LVEF for all SAD and MAD subjects across a broad range of EDG-7500 exposures. In the MAD, a half-life of approximately 30 hours was observed, and steady state was achieved in approximately 4 days after the start of once-daily dosing. Generally, dose proportional increases in exposure were observed in both SAD and MAD.
In CIRRUS-HCM Part A, patients with obstructive HCM received a single dose of 50, 100 or 200 mg of EDG-7500. A
Across the Phase 1 and CIRRUS-HCM trials, no subjects had a LVEF reduction to below
“Based on the strength of clinical and preclinical data to-date, we have initiated the 28-day part of CIRRUS-HCM in patients with obstructive and non-obstructive HCM,” said Marc Semigran, M.D., Chief Development Officer, Edgewise Therapeutics. “Importantly, we plan to continue the evaluation of tolerability, pharmacokinetics and effects on LVOT-G, LVEF, biomarkers and measures of feel and function in these patients.”
Anjali T. Owens, M.D., Medical Director, Center for Inherited Cardiac Disease, Associate Professor of Medicine, University of
Kevin Koch, Ph.D.,
EDG-7500 Topline Data Webcast Event
Members of the Edgewise management team will hold a live webcast on Thursday, September 19, 2024, at 8:30 am ET to discuss the top-line data, and will be joined by leading cardiology expert, Anjali T. Owens, M.D., Medical Director, Center for Inherited Cardiac Disease, Associate Professor of Medicine, University of
About EDG-7500
EDG-7500 is a novel oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with hypertrophic cardiomyopathy (HCM) and other diseases of diastolic dysfunction. Preclinical data in models of both obstructive and non-obstructive HCM suggest the ability to drive a beneficial response at a low risk of decreasing left ventricular ejection fraction below normal. The Company is enrolling CIRRUS-HCM, a three-part, multi-center, open-label trial, in approximately 55 patients with HCM at up to 20 clinical sites in the
To learn more about CIRRUS-HCM, visit clinicaltrials.gov, NCT06347159 (Phase 2).
About Hypertrophic Cardiomyopathy
Hypertrophic Cardiomyopathy (HCM) is the most common form of genetic heart disease, affecting approximately one in 500 people, and is associated with reduced quality of life and an elevated risk of heart failure, abnormal heart rhythms, and sudden cardiac death (SCD). Individuals with HCM can become extremely limited in their functional capacity and ability to perform the activities of daily living. Commonly experienced symptoms include breathlessness, irregular heartbeats, chest pain, tiredness, dizziness, or even fainting. These symptoms are caused by excessive contraction and thickening (hypertrophy) of the left ventricular wall of the heart. Over time, the thickened muscle becomes stiff, making it difficult for the heart to relax and fill with blood (diastolic dysfunction). There are two major forms of HCM obstructive and non-obstructive. The obstructive HCM pathology is observed in two thirds, while non-obstructive HCM is present in one third of all individuals with HCM. Despite advancements in treatment options for some HCM patients, there remains a significant unmet need for additional therapeutic approaches for patients.
About Edgewise Therapeutics
Edgewise Therapeutics is a leading muscle disease biopharmaceutical company developing novel therapeutics for muscular dystrophies and serious cardiac conditions. The Company’s deep expertise in muscle physiology is driving a new generation of novel therapeutics. Sevasemten is an orally administered skeletal myosin inhibitor in late-stage clinical trials in Becker and Duchenne muscular dystrophies. EDG-7500 is a novel cardiac sarcomere modulator for the treatment of hypertrophic cardiomyopathy and other diseases of diastolic dysfunction, currently in Phase 2 clinical development. The entire team at Edgewise is dedicated to our mission: changing the lives of patients and families affected by serious muscle diseases. To learn more, go to: www.edgewisetx.com or follow us on LinkedIn, X, Facebook and Instagram.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, statements regarding the potential of, and expectations regarding EDG-7500; statements regarding the timing of reporting data (including the data from the CIRRUS-HCM 28-day trial); statements regarding Edgewise’s expectations relating to its clinical trials (including the Phase 2 trial of EDG-7500 in individuals with obstructive HCM, the CIRRUS-HCM 28-day trial, and open-label extension trial of EDG-7500); statements regarding the commencement of trials (including the open-label extension trial of EDG-7500); and statements by Edgewise’s president and chief executive officer and chief development officer. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. The forward-looking statements contained herein are based upon Edgewise’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those projected in any forward-looking statements due to numerous risks and uncertainties, including but not limited to: risks associated with Edgewise’s limited operating history, its products being early in development and not having products approved for commercial sale; risks associated with Edgewise not having generated any revenue to date; Edgewise’s ability to achieve objectives relating to the discovery, development and commercialization of its product candidates, if approved; Edgewise’s need for substantial additional capital to finance its operations; Edgewise’s substantial dependence on the success of its sevasemten; Edgewise’s ability to develop and commercialize sevasemten and EDG-7500 and discover, develop and commercialize product candidates in future programs; risks related to Edgewise’s clinical trials of its product candidates not demonstrating safety and efficacy; risks related to Edgewise’s product candidates causing serious adverse events, toxicities or other undesirable side effects; the outcome of preclinical testing and early clinical trials not being predictive of the success of later clinical trials and the risks related to the results of Edgewise’s clinical trials not satisfying the requirements of regulatory authorities; delays or difficulties in the enrollment and/or maintenance of patients in clinical trials; risks related to failure to capitalize on other indications or product candidates; risks related to competition; risks relating to interim, topline and preliminary data from Edgewise’s clinical trials changing as more patient data becomes available; risks related to the regulatory approval processes being lengthy, time consuming and inherently unpredictable; risks related to regulatory authorities not accepting data from trials conducted in locations outside of their jurisdiction; risks relating to Edgewise’s ability to attract and retain highly skilled executive officers and employees; Edgewise’s ability to obtain and maintain intellectual property protection for its product candidates; Edgewise’s reliance on third parties; general economic and market conditions; and other risks. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in documents that Edgewise files from time to time with the
This press release contains hyperlinks to information that is not deemed to be incorporated by reference into this press release.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240919115598/en/
Investors:
Michael Carruthers, Chief Financial Officer
ir@edgewisetx.com
Media:
Maureen Franco, VP Corporate Communications
media@edgewisetx.com
Source: Edgewise Therapeutics







