EDWARDS EVOQUE TRANSCATHETER TRICUSPID VALVE REPLACEMENT SYSTEM RECEIVES CE MARK
- The EVOQUE system is the world's first transcatheter valve replacement therapy to receive regulatory approval to treat tricuspid regurgitation (TR).
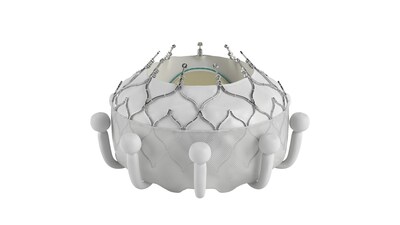
- The EVOQUE system is able to fully replace the tricuspid valve, virtually eliminating tricuspid regurgitation in a wide range of anatomies.
- One-year results from the TRISCEND study showed high survival (90.1%) and high freedom from heart failure hospitalization (88.4%).
- Significant and sustained TR reduction to mild or trace TR (97.6%) was observed with the EVOQUE system.
- The company will present results from the TRISCEND II pivotal trial during the 35th Transcatheter Cardiovascular Therapeutics (TCT) symposium.
- The company does not yet have any transcatheter therapies approved for treatment of the tricuspid valve in the United States.
Insights
Analyzing...
"Innovating for unmet patient needs is at the center of everything we do at Edwards, which makes us especially proud to have received CE Mark for this first-of-its-kind transcatheter tricuspid valve replacement therapy," said Daveen Chopra, Edwards' corporate vice president, transcatheter mitral and tricuspid therapies. "With the EVOQUE system's approval, in addition to our current PASCAL tricuspid system, we are now able to provide a broader array of much-needed treatment options for appropriate tricuspid disease patients in
The EVOQUE system is comprised of a nitinol self-expanding frame, intra-annular sealing skirt, and tissue leaflets made from the same bovine pericardial tissue as the company's market-leading heart valves. The EVOQUE valve will be available in three sizes, all delivered through a low-profile transfemoral 28F system.
"The EVOQUE system is able to fully replace the tricuspid valve, virtually eliminating tricuspid regurgitation in a wide range of anatomies," said Prof. Philipp Lurz, Director of Cardiology, University of Mainz,
One-year results on patients treated in the single-arm, prospective, global, multi-center TRISCEND study of the EVOQUE system were presented at PCR London Valves 2022 and demonstrated favorable safety and effectiveness outcomes and significant quality-of-life improvements. Key findings included high survival (
The company will present results from the TRISCEND II pivotal trial, studying the EVOQUE system, during a late-breaking clinical trial session on Oct. 26 in
Patients with tricuspid valve disease suffer greatly with symptoms ranging from debilitating to life-threatening with few effective options for relief. Other transcatheter therapies in Edwards' tricuspid portfolio with CE Mark approval include the PASCAL Precision transcatheter repair system and the Cardioband annular reduction system. The company does not yet have any transcatheter therapies approved for treatment of the tricuspid valve in
About Edwards Lifesciences
Edwards Lifesciences is the global leader of patient-focused innovations for structural heart disease and critical care monitoring. We are driven by a passion for patients, dedicated to improving and enhancing lives through partnerships with clinicians and stakeholders across the global healthcare landscape. For more information, visit Edwards.com and follow us on Facebook, Instagram, LinkedIn, X and YouTube.
This news release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, but are not limited to, statements made by Mr. Chopra and statements regarding expected product benefits, patient outcomes, objectives and expectations and other statements that are not historical facts. Forward-looking statements are based on estimates and assumptions made by management of the company and are believed to be reasonable, though they are inherently uncertain and difficult to predict. Our forward-looking statements speak only as of the date on which they are made, and we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of the statement. Investors are cautioned not to unduly rely on such forward-looking statements.
Forward-looking statements involve risks and uncertainties that could cause results to differ materially from those expressed or implied by the forward-looking statements based on a number of factors as detailed in the company's filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2022, and its Quarterly Reports on Form 10-Q for the quarters ended March 31 and June 30, 2023. These filings, along with important safety information about our products, may be found at Edwards.com.
Edwards, Edwards Lifesciences, the stylized E logo, Cardioband, Edwards EVOQUE, EVOQUE, PASCAL, PASCAL Precision, and TRISCEND are trademarks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/edwards-evoque-transcatheter-tricuspid-valve-replacement-system-receives-ce-mark-301961543.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/edwards-evoque-transcatheter-tricuspid-valve-replacement-system-receives-ce-mark-301961543.html
SOURCE Edwards Lifesciences Corporation