Evoke Pharma & EVERSANA Announce Statistically Significant Improvement in Patient Outcomes for GLP-1 users with Diabetic Gastroparesis using GIMOTI®
Evoke Pharma announced significant improvements in patient outcomes for GLP-1 users with diabetic gastroparesis using GIMOTI nasal spray compared to oral metoclopramide. A real-world study of 92 patients showed GIMOTI users experienced 91% fewer emergency department visits, 41% fewer office visits, and 89% fewer hospital outpatient visits within a 6-month period. The study, presented at ACG 2024, received both the Presidential Poster Award and Outstanding Research Award in the Stomach Category. The research demonstrates GIMOTI's potential as supportive care for GLP-1 therapy, with no serious adverse events reported in a separate safety analysis.
Evoke Pharma ha annunciato miglioramenti significativi negli esiti dei pazienti con gastroparesi diabetica che utilizzano GLP-1, impiegando lo spray nasale GIMOTI rispetto al metoclopramide orale. Uno studio nel mondo reale condotto su 92 pazienti ha dimostrato che gli utenti di GIMOTI hanno registrato il 91% in meno di visite al pronto soccorso, il 41% in meno di visite ambulatoriali e l'89% in meno di visite ospedaliere specialistiche in un periodo di 6 mesi. Lo studio, presentato all'ACG 2024, ha ricevuto sia il Presidential Poster Award che l'Oustanding Research Award nella Categoria Stomaco. La ricerca dimostra il potenziale di GIMOTI come supporto alla terapia GLP-1, senza eventi avversi seri segnalati in un'analisi di sicurezza separata.
Evoke Pharma anunció mejoras significativas en los resultados de los pacientes con gastroparesis diabética que utilizan GLP-1, utilizando el aerosol nasal GIMOTI en comparación con el metoclopramida oral. Un estudio en el mundo real con 92 pacientes mostró que los usuarios de GIMOTI experimentaron un 91% menos de visitas a la sala de emergencias, un 41% menos de visitas al consultorio y un 89% menos de visitas ambulatorias en el hospital durante un período de 6 meses. El estudio, presentado en ACG 2024, recibió tanto el Presidential Poster Award como el Outstanding Research Award en la categoría de Estómago. La investigación demuestra el potencial de GIMOTI como cuidado de apoyo para la terapia de GLP-1, sin eventos adversos graves reportados en un análisis de seguridad separado.
Evoke Pharma는 GLP-1 사용자 중 당뇨병성 위마비 환자들이 경구용 메토클로프라미드에 비해 GIMOTI 비강 스프레이를 사용했을 때 환자의 결과가 상당히 개선되었다고 발표했습니다. 92명의 환자를 대상으로 한 실제 연구에서는 GIMOTI 사용자가 6개월 동안 응급실 방문이 91% 감소, 진료소 방문이 41% 감소, 병원 외래 진료 방문이 89% 감소했음을 보여주었습니다. ACG 2024에서 발표된 이 연구는 Presidential Poster Award와 Stomach Category에서 Outstanding Research Award를 수상했습니다. 이 연구는 GLP-1 요법을 위한 지원 치료로서 GIMOTI의 잠재력을 입증하며, 별도의 안전성 분석에서 심각한 부작용이 보고되지 않았습니다.
Evoke Pharma a annoncé des améliorations significatives des résultats des patients utilisant GLP-1 avec une gastroparesie diabétique utilisant le spray nasal GIMOTI par rapport au métoclopramide oral. Une étude réalisée avec 92 patients a montré que les utilisateurs de GIMOTI ont connu 91% de visites en moins aux urgences, 41% de visites en moins au cabinet médical et 89% de visites en moins aux consultations externes de l'hôpital sur une période de 6 mois. L'étude, présentée à l'ACG 2024, a reçu à la fois le Presidential Poster Award et le Outstanding Research Award dans la catégorie Estomac. La recherche démontre le potentiel de GIMOTI comme soin d'accompagnement pour la thérapie GLP-1, sans événements indésirables graves rapportés dans une analyse de sécurité distincte.
Evoke Pharma gab bekannt, dass es signifikante Verbesserungen bei den Behandlungsergebnissen von Patienten mit diabetischer Gastroparese gibt, die GLP-1 nutzen und das GIMOTI Nasenspray im Vergleich zu oralem Metoclopramid verwenden. Eine realweltliche Studie mit 92 Patienten zeigte, dass GIMOTI-Nutzer innerhalb von 6 Monaten 91% weniger Notaufnahmen, 41% weniger Arztbesuche und 89% weniger ambulante Krankenhausbesuche hatten. Die Studie, die auf der ACG 2024 präsentiert wurde, erhielt sowohl den Presidential Poster Award als auch den Outstanding Research Award in der Kategorie Magen. Die Forschung zeigt das Potenzial von GIMOTI als unterstützende Pflege bei der GLP-1-Therapie, wobei in einer separaten Sicherheitsanalyse keine schwerwiegenden unerwünschten Ereignisse berichtet wurden.
- 91% reduction in emergency department visits vs oral metoclopramide (p=0.001)
- 89% reduction in hospital outpatient visits (p=0.032)
- 41% reduction in office visits (p=0.027)
- Healthcare cost reduction of over $15,000 per patient in six months vs oral metoclopramide
- Received Presidential Poster Award (top 5% of conference data) and Outstanding Research Award
- None.
Insights
This real-world data study reveals significant clinical benefits for GIMOTI over oral metoclopramide in patients using GLP-1 medications. The results show remarkable reductions in healthcare utilization, including
The safety profile in the female subgroup analysis showed no serious adverse events, strengthening GIMOTI's position as a supportive therapy for GLP-1 users. The
This data significantly strengthens GIMOTI's market position in the rapidly expanding GLP-1 market. With major GLP-1 drugs like Wegovy and Ozempic seeing widespread adoption, GIMOTI's proven effectiveness in managing associated gastroparesis creates a valuable market opportunity. The dual benefits of improved patient outcomes and reduced healthcare costs create a compelling value proposition for both insurers and healthcare providers.
The Presidential Poster Award recognition adds credibility and could accelerate physician adoption. For EVOK shareholders, this positions the company to capture a growing segment of patients experiencing GI complications from GLP-1 use, potentially driving significant revenue growth.
Analysis of real-world data compared patients on GIMOTI (n=51) to Oral Metoclopramide (n=41), both taking GLP-1s, showing significant statistical improvement for GIMOTI over Oral Metoclopramide in All Cause Emergency Department Visits (-
Data presented at American College of Gastroenterology (ACG) 2024; the submission garnered both the Presidential Poster Award as one of the top
First study to show Gimoti’s potential as supportive care for GLP-1 therapy
SOLANA BEACH, Calif., Oct. 28, 2024 (GLOBE NEWSWIRE) -- Evoke Pharma, Inc. (NASDAQ: EVOK), a specialty pharmaceutical company focused on developing treatments for gastrointestinal (GI) diseases, with a particular emphasis on GIMOTI® (metoclopramide) nasal spray, together with EVERSANA, a leading provider of global commercial services to the life sciences industry, today announced the presentation of data for GLP-1 users with diabetic gastroparesis using GIMOTI at the American College of Gastroenterology (ACG) 2024 Annual Meeting.
The real-world retrospective study evaluated the impact of GIMOTI (metoclopramide nasal spray) in patients with diabetic gastroparesis (DGP) who were concurrently using GLP-1 receptor agonists. GLP-1 drugs are commonly prescribed for type 2 diabetes, and with the approval of GLP-1 medications, there have been increasing reports of these drugs exacerbating GI symptoms, specifically gastroparesis. GLP-1 medications function, in part, by causing delayed gastric emptying. Delayed gastric emptying, in the absence of mechanical obstruction, is the definition of gastroparesis. In a recent National Institute of Health (NIH) study (https://pubmed.ncbi.nlm.nih.gov/38399414/), an estimated
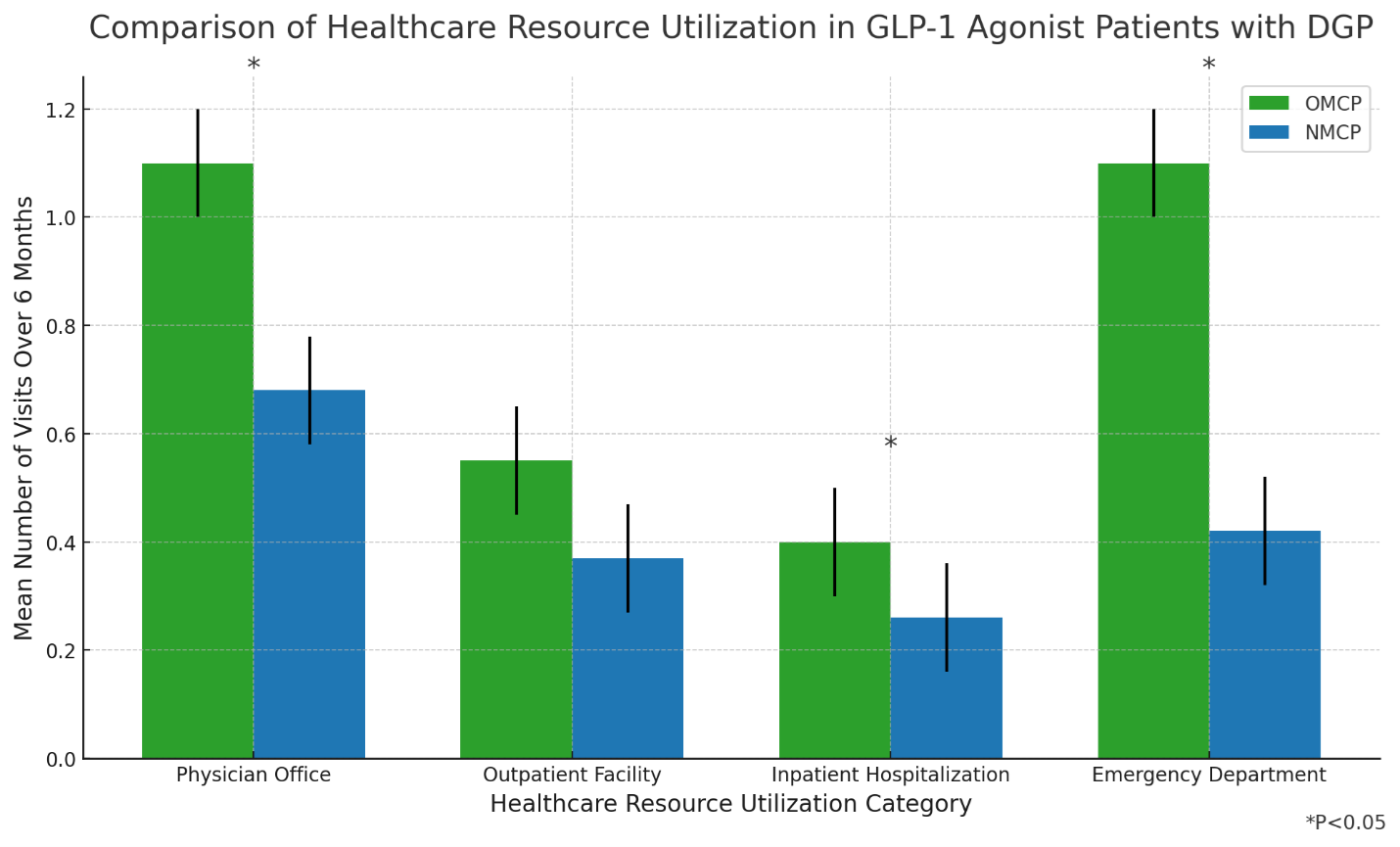
The GIMOTI study compared healthcare resource utilization (HRU) between patients using GIMOTI nasal metoclopramide (NMCP) and those on oral metoclopramide (OMCP), specifically focusing on individuals with a prior GLP-1 prescription. Significant reductions across a collection of outcomes were observed in patients treated with GIMOTI versus oral metoclopramide.
Key Study Findings Presented at ACG 2024:
- Patients with prior GLP-1 history had reduced HRU after taking NMCP
- In NMCP patients, all-cause emergency department (ED) visits decreased by
55% (mean [SD]: 0.25 [1.13] post-index vs. 0.55 [1.30] pre-index; p=0.063); and - DGP-related ED visits decreased by
28% (mean [SD]: 0.18 [0.99] post-index vs. 0.25 [1.28] pre-index; p=0.203).
- In NMCP patients, all-cause emergency department (ED) visits decreased by
- In patients taking GLP-1, those that took NMCP had fewer healthcare visits compared to those taking OMCP
- All-cause and DGP-related ED visits were
91% lower (cIRR: 0.09,95% CI: 0.01, 0.42; p=0.001) and89% lower (cIRR: 0.11,95% CI: 0, 0.93; p=0.046) for NMCP vs. OMCP; and - All-cause Hospital Outpatient visits were
89% lower (cIRR = 0.11,95% CI: 0.01, 0.73; p = 0.032) - All-cause and DGP-related office visits were
41% lower (cIRR: 0.59,95% CI: 0.37, 0.94; p=0.027) and66% lower (cIRR: 0.34,95% CI: 0.017, 0.65; p=0.001) for NMCP vs. OMCP.
- All-cause and DGP-related ED visits were
- NMCP can be used to effectively treat patients with gastroparesis taking GLP-1 treatment and avoid costly healthcare visits
- All-cause clinic, outpatient, and inpatient visits showed similar trends favoring NMCP vs. OMCP.
- In DGP patients with a prior claim for GLP-1, NMCP use was associated with numerically and significantly reduced all-cause and DGP-related HRU compared to pre-treatment utilization and OMCP-treated controls.
"This data is particularly encouraging given the rising number of patients using GLP-1 agonists for diabetes, many of whom also suffer from gastroparesis," said Matt D’Onofrio, CEO of Evoke Pharma. "Our goal is to provide data regarding Gimoti as supportive care for diabetic gastroparesis for those also on GLP-1 agonists. This study, combined with the earlier real-world data presented at key conferences, reinforces GIMOTI's potential to improve outcomes for patients and reduce the overall financial burden on the healthcare system."
The study's specific cohort consisted of 92 total patients between the nasal metoclopramide (NMCP, N=51) and oral metoclopramide (OMCP, N=41) groups. The NMCP group had a slightly older average age (55.1 years) compared to the OMCP group (53.1 years). A notable portion of the NMCP group (
In a separate study, a post-hoc analysis focused on safety data for a female subgroup from a previous Phase 3 study that included 36 women using GLP-1 drugs during the 28-day study. Among these, 13 were treated with GIMOTI and 23 with placebo. The majority (
"Our continued focus is helping patients that have gastroparesis and diabetes. The fact that up to 20 million patients (KFF Health Tracking Poll (April 23-May 1, 2024) (kff.org/KFF Health Tracking Poll)) may have taken or are currently taking GLP-1s for their diabetes, indicates this is an important patient constituency the medical community needs to monitor and address," said Chris Quesenberry, Chief Commercial Officer of Gimoti. "Evoke Pharma, through its clinical development program and real-world evidence studies, is uniquely positioned to provide clinicians with information that may inform treatment decisions. This study provides insight that clinicians who treated their patients with diabetic gastroparesis, who were also taking a GLP-1, benefitted in taking GIMOTI®, an innovative, non-oral therapy that does not rely on a dysfunctional gut to work."
The results presented at ACG 2024 build on a growing body of real-world data that underscores GIMOTI's ability to significantly reduce HRU in patients with diabetic gastroparesis. Earlier studies presented at Digestive Disease Week (DDW) 2024 and ACG 2023 demonstrated that patients treated with GIMOTI showed fewer hospitalizations, emergency department visits, and physician office visits compared to those using oral metoclopramide. Also, these studies highlighted that GIMOTI reduced healthcare costs by over
"These studies further confirm the important role of GIMOTI in managing diabetic gastroparesis and supports its use for patients concurrently on GLP-1 agonists," said Dr. Michael Cline, Medical Director of the Gastroparesis Clinic at Cleveland Clinic, an author of the study. "In my practice, I’ve treated patients arriving with gastroparesis while on a GLP-1. This data demonstrates a significant reduction in emergency room visits, office visits, and overall healthcare resource utilization, which not only improves patient outcomes but also reduces the strain on healthcare systems. The nasal delivery of metoclopramide offers a distinct advantage for patients with delayed gastric emptying, ensuring they receive the relief they need without the complications or unknown absorption associated with oral medications."
EVERSANA was named Evoke’s commercialization partner to support commercialization efforts of GIMOTI, first in 2020 and then an extended partnership was announced in February 2022.
Slides referencing data to the studies can be found under news and presentations of the Evoke Pharma investor relations website.
About Evoke Pharma, Inc.
Evoke is a specialty pharmaceutical company focused primarily on the development of drugs to treat GI disorders and diseases. The company developed, commercialized and markets GIMOTI, a nasal spray formulation of metoclopramide, for the relief of symptoms associated with acute and recurrent diabetic gastroparesis in adults.
Diabetic gastroparesis is a GI disorder affecting millions of patients worldwide, in which the stomach takes too long to empty its contents resulting in serious GI symptoms as well as other systemic complications. The gastric delay caused by gastroparesis can compromise absorption of orally administered medications. Prior to FDA approval to commercially market GIMOTI, metoclopramide was only available in oral and injectable formulations and remains the only drug currently approved in the United States to treat gastroparesis.
Visit www.EvokePharma.com for more information.
Follow Evoke Pharma on LinkedIn
Follow Evoke Pharma on Twitter
About Gimoti® (metoclopramide) nasal spray
GIMOTI is indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. Important Safety Information:
WARNING: TARDIVE DYSKINESIA
- Metoclopramide can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. The risk of developing TD increases with duration of treatment and total cumulative dosage.
- Discontinue GIMOTI in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after metoclopramide is stopped.
- Avoid treatment with metoclopramide (all dosage forms and routes of administration) for longer than 12 weeks because of the increased risk of developing TD with longer-term use.
GIMOTI is not recommended for use in:
- Pediatric patients due to the risk of developing tardive dyskinesia (TD) and other extrapyramidal symptoms as well as the risk of methemoglobinemia in neonates.
- Moderate or severe hepatic impairment (Child-Pugh B or C), moderate or severe renal impairment (creatinine clearance less than 60 mL/minute), and patients concurrently using strong CYP2D6 inhibitors due to the risk of increased drug exposure and adverse reactions.
GIMOTI is contraindicated:
- In patients with a history of tardive dyskinesia (TD) or a dystonic reaction to metoclopramide.
- When stimulation of gastrointestinal motility might be dangerous (e.g., in the presence of gastrointestinal hemorrhage mechanical obstruction, or perforation).
- In patients with pheochromocytoma or other catecholamine-releasing paragangliomas. Metoclopramide may cause a hypertensive/pheochromocytoma crisis, probably due to release of catecholamines from the tumor.
- In patients with epilepsy. Metoclopramide may increase the frequency and severity of seizures.
- In patients with hypersensitivity to metoclopramide. Reactions have included laryngeal and glossal angioedema and bronchospasm.
Potential adverse reactions associated with metoclopramide include: Tardive dyskinesia (TD), other extrapyramidal effects (EPS), parkinsonism symptoms, motor restlessness, neuroleptic malignant syndrome (NMS), depression, suicidal ideation and suicide, hypertension, fluid retention, hyperprolactinemia, effects on the ability to drive and operate machinery. Most common adverse reactions (≥
Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Safe Harbor Statement
Evoke cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negatives of these terms or other similar expressions. These statements are based on the company’s current beliefs and expectations. These forward-looking statements include statements regarding: GIMOTI’s potential to reduce HRU by diabetic gastroparesis patents taking GLP-1 therapies; the possibility that increased use of GLP-1 agonists could increase the need for treatment of gastroparesis or increase the patient base for GIMOTI; and Evoke’s belief that GIMOTI can improve treatment of gastroparesis in patients taking GLP-1 agonists. The inclusion of forward-looking statements should not be regarded as a representation by Evoke that any of its plans will be achieved. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in Evoke’s business, including, without limitation: Evoke and EVERSANA may not be able to successfully drive market demand for GIMOTI; the results of market research studies may not predict acceptance by patients, healthcare providers or payors; GLP-1 agonists may not increase the number of patients diagnosed with gastroparesis, which remains speculative; alternative treatments for gastroparesis may be developed or approved and may be shown to be superior to GIMOTI; Evoke’s ability to obtain additional financing as needed to support its operations; Evoke may use its capital resources sooner than expected; Evoke’s ability to maintain compliance with Nasdaq’s stockholder’s equity requirements; Evoke’s dependence on third parties for the manufacture of GIMOTI; Evoke is entirely dependent on the success of GIMOTI; inadequate efficacy or unexpected adverse side effects relating to GIMOTI that could result in recalls or product liability claims; Evoke’s ability to maintain intellectual property protection for GIMOTI; and other risks and uncertainties detailed in Evoke’s prior press releases and in the periodic reports it files with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Evoke undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
About EVERSANA
EVERSANA® is a leading independent provider of global services to the life sciences industry. The company’s integrated solutions are rooted in the patient experience and span all stages of the product life cycle to deliver long-term, sustainable value for patients, prescribers, channel partners and payers. The company serves more than 650 organizations, including innovative start-ups and established pharmaceutical companies, to advance life sciences solutions for a healthier world. To learn more about EVERSANA, visit eversana.com or connect through LinkedIn and X.
Investor & Media Contact:
Daniel Kontoh-Boateng
DKB Partners
Tel: 862-213-1398
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/cfc46127-a976-498b-8e19-d5c879171b81








