Equillium Announces Positive Data from Phase 2 Study Evaluating Itolizumab in Patients with Moderate to Severe Ulcerative Colitis
Equillium (NASDAQ: EQ) announced positive topline results from its Phase 2 study of itolizumab in treating moderate to severe ulcerative colitis (UC). The study involved 90 biologic-naïve patients randomized 1:1:1 to receive itolizumab, placebo, or adalimumab.
Key findings after 12 weeks of treatment include:
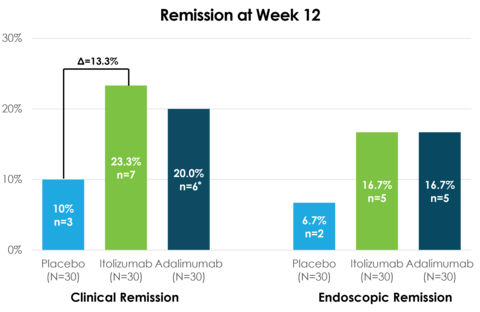
- Clinical remission rate: 23.3% for itolizumab vs 20.0% for adalimumab and 10.0% for placebo
- Clinical response rate: 63.3% for itolizumab vs 60.0% for adalimumab and 46.7% for placebo
- Endoscopic remission: 16.7% for both itolizumab and adalimumab vs 6.7% for placebo
The drug was generally well tolerated, showing comparable efficacy to adalimumab, despite having more severe patients in the itolizumab arm at baseline. Additional data is expected to be presented at a scientific conference during 2025.
Equillium (NASDAQ: EQ) ha annunciato risultati positivi preliminari dal suo studio di Fase 2 su itolizumab per il trattamento della colite ulcerosa (UC) da moderata a grave. Lo studio ha coinvolto 90 pazienti mai trattati con biologici, randomizzati in un rapporto 1:1:1 per ricevere itolizumab, placebo o adalimumab.
I risultati chiave dopo 12 settimane di trattamento includono:
- Percentuale di remissione clinica: 23,3% per itolizumab contro 20,0% per adalimumab e 10,0% per placebo
- Percentuale di risposta clinica: 63,3% per itolizumab contro 60,0% per adalimumab e 46,7% per placebo
- Remissione endoscopica: 16,7% sia per itolizumab che per adalimumab contro 6,7% per placebo
Il farmaco è stato generalmente ben tollerato, mostrando un'efficacia comparabile ad adalimumab, nonostante ci fossero pazienti più gravi nel gruppo itolizumab all'inizio. Ulteriori dati sono attesi per essere presentati a una conferenza scientifica nel 2025.
Equillium (NASDAQ: EQ) anunció resultados positivos preliminares de su estudio de Fase 2 sobre itolizumab para el tratamiento de la colitis ulcerosa (UC) de moderada a grave. El estudio incluyó a 90 pacientes sin tratamiento biológico, aleatorizados en una relación 1:1:1 para recibir itolizumab, placebo o adalimumab.
Los hallazgos clave después de 12 semanas de tratamiento incluyen:
- Tasa de remisión clínica: 23,3% para itolizumab frente a 20,0% para adalimumab y 10,0% para placebo
- Tasa de respuesta clínica: 63,3% para itolizumab frente a 60,0% para adalimumab y 46,7% para placebo
- Remisión endoscópica: 16,7% tanto para itolizumab como para adalimumab frente a 6,7% para placebo
El medicamento fue generalmente bien tolerado, mostrando una eficacia comparable a la de adalimumab, a pesar de que había pacientes más graves en el grupo de itolizumab al inicio. Se espera que se presenten datos adicionales en una conferencia científica en 2025.
Equillium (NASDAQ: EQ)는 중증에서 심각한 궤양성 대장염(UC) 치료를 위한 itolizumab의 2상 연구에서 긍정적인 결과를 발표했습니다. 이 연구는 생물학적 치료를 받은 적이 없는 90명의 환자를 대상으로 itolizumab, 위약 또는 아달리무맙을 1:1:1로 무작위 배정했습니다.
12주 치료 후 주요 발견은 다음과 같습니다:
- 임상 관해율: itolizumab 23.3%, 아달리무맙 20.0%, 위약 10.0%
- 임상 반응률: itolizumab 63.3%, 아달리무맙 60.0%, 위약 46.7%
- 내시경적 관해: itolizumab 및 아달리무맙 모두 16.7%, 위약 6.7%
이 약물은 일반적으로 잘 견디며, 아달리무맙과 유사한 효능을 보였고 이톨리주맙 그룹의 환자들이 기본적으로 더 심각한 상태였음에도 불구하고 효과적이었습니다. 추가 데이터는 2025년에 열리는 학술 회의에서 발표될 예정입니다.
Equillium (NASDAQ: EQ) a annoncé des résultats préliminaires positifs de son étude de Phase 2 sur l'itolizumab pour le traitement de la colite ulcéreuse (UC) modérée à sévère. L'étude a impliqué 90 patients n'ayant jamais reçu de traitement biologique, randomisés dans un rapport de 1:1:1 pour recevoir soit de l'itolizumab, un placebo ou de l'adalimumab.
Les résultats clés après 12 semaines de traitement incluent :
- Taux de rémission clinique : 23,3 % pour l'itolizumab contre 20,0 % pour l'adalimumab et 10,0 % pour le placebo
- Taux de réponse clinique : 63,3 % pour l'itolizumab contre 60,0 % pour l'adalimumab et 46,7 % pour le placebo
- Rémission endoscopique : 16,7 % pour l'itolizumab et l'adalimumab contre 6,7 % pour le placebo
Le médicament a été généralement bien toléré, montrant une efficacité comparable à celle de l'adalimumab, malgré la présence de patients plus graves dans le bras d'itolizumab au départ. Des données supplémentaires devraient être présentées lors d'une conférence scientifique en 2025.
Equillium (NASDAQ: EQ) hat positive vorläufige Ergebnisse aus seiner Phase-2-Studie zu Itolizumab bei der Behandlung von moderater bis schwerer kolorektaler Entzündung (UC) bekannt gegeben. Die Studie umfasste 90 biologisch naive Patienten, die im Verhältnis 1:1:1 randomisiert wurden, um Itolizumab, einen Placebo oder Adalimumab zu erhalten.
Wichtige Ergebnisse nach 12 Wochen Behandlung sind:
- Klinische Remissionsrate: 23,3% für Itolizumab gegenüber 20,0% für Adalimumab und 10,0% für Placebo
- Klinische Ansprechrate: 63,3% für Itolizumab gegenüber 60,0% für Adalimumab und 46,7% für Placebo
- Endoskopische Remission: 16,7% sowohl für Itolizumab als auch für Adalimumab gegenüber 6,7% für Placebo
Das Medikament wurde allgemein gut vertragen, zeigte eine vergleichbare Wirksamkeit wie Adalimumab, obwohl die Patienten in der Itolizumab-Gruppe zu Beginn schwerer waren. Weitere Daten werden voraussichtlich auf einer wissenschaftlichen Konferenz im Jahr 2025 präsentiert.
- Achieved 23.3% clinical remission rate, outperforming both adalimumab (20.0%) and placebo (10.0%)
- Demonstrated 63.3% clinical response rate, higher than adalimumab (60.0%) and placebo (46.7%)
- Matched adalimumab's endoscopic remission rate of 16.7%, exceeding placebo (6.7%)
- Drug showed favorable safety profile with no safety signals observed
- Baseline disease severity was imbalanced, with 23% severe cases in itolizumab arm vs 0% in other arms
- Full efficacy data beyond 12 weeks not yet available
Insights
The Phase 2 results for itolizumab represent a significant milestone for Equillium, demonstrating competitive efficacy in ulcerative colitis treatment. The 23.3% clinical remission rate is particularly impressive considering the study arm included a higher proportion of severe cases (23% with Total Mayo Score of 11), potentially understating the drug's true efficacy in a balanced patient population.
Several key factors enhance the strategic value of these results:
- The comparable efficacy to adalimumab, a well-established $20 billion revenue-generating biologic, positions itolizumab as a viable alternative in the competitive IBD market
- The novel CD6-ALCAM pathway mechanism offers potential differentiation in a crowded market seeking alternative treatment approaches
- The favorable safety profile supports potential combination therapy opportunities
- The results strengthen the broader clinical program, particularly supporting the ongoing Phase 3 EQUATOR study in acute graft-versus-host disease
The data suggests significant commercial potential in the $7.5 billion ulcerative colitis market. The demonstrated efficacy in more severe cases could position itolizumab as a preferred option for difficult-to-treat patients. The upcoming Phase 3 EQUATOR study results this quarter could serve as a major catalyst for Equillium's market valuation, potentially leading to partnership opportunities or increased institutional investment interest.
Itolizumab demonstrated clinical efficacy after 12 weeks of treatment, achieving a clinical remission rate of
Itolizumab achieved key secondary endpoint of endoscopic remission of
Itolizumab was generally well tolerated consistent with prior clinical experience
(Graphic: Business Wire)
The double-blinded, placebo- and active-controlled Phase 2 clinical study evaluated the safety and efficacy of itolizumab in biologic-naïve patients with moderate to severe active UC. A total of 90 patients were randomized 1:1:1 to receive itolizumab (fixed dose of 140 mg), placebo, or adalimumab (a global standard of care biologic treatment used as an active control) every two weeks for an initial 12-week treatment period. The primary endpoint of the study was clinical remission as defined by Total Mayo Score, and secondary endpoints included the proportion of participants who achieved clinical response and endoscopic remission (evaluated by central endoscopy). The study was co-sponsored by Equillium and Biocon Limited and conducted at multiple clinical trial sites in
“The CD6-ALCAM pathway is elevated in gastrointestinal inflammation and is associated with severity of disease in both ulcerative colitis and Crohn’s patients. As such, we are delighted with the strength of data across the primary and secondary endpoints of this Phase 2 study in moderate to severe ulcerative colitis patients,” said Dr. Stephen Connelly, chief scientific officer at Equillium. “Itolizumab was well tolerated and achieved a clinical remission rate of 23 percent despite an imbalance of more severe patients in the itolizumab arm compared to the other arms of the study. While these positive results add to itolizumab’s critical mass of safety and efficacy data across different patient populations, we are particularly encouraged by this data in the context of our Phase 3 EQUATOR study in acute graft-versus-host disease, where lower gastrointestinal pathogenesis is a key driver of mortality, with topline data expected this quarter.”
“Itolizumab demonstrated proof of concept with a meaningful effect size – comparable to biologic standard of care adalimumab – in this Phase 2 study in subjects with moderate to severe ulcerative colitis,” said Dr. Brian Feagan, Professor of Medicine at the Schulich School of Medicine & Dentistry at the University of
Summary of Key Study Results
Baseline demographics of the study included a median age of 39 years, relatively equal proportions of male and female subjects evenly distributed among study arms, and a mean weight of 58 kilograms.
Baseline disease severity of the study was greater in the itolizumab arm, where
The primary endpoint of the study was clinical remission, defined as Total Mayo Score of ≤ 2 with no individual sub-score greater than 1 at Week 12. Secondary endpoints included the proportion of participants who achieved clinical response (per Total Mayo Score) and endoscopic remission (evaluated by central endoscopy) at Week 12 and Week 24. Additional data is expected to be presented at a future scientific conference during 2025.
-
23.3% clinical remission in the itolizumab arm at 12 weeks (primary endpoint) vs.20.0% in adalimumab* vs.10.0% in placebo -
63.3% clinical response in the itolizumab arm at 12 weeks vs.60.0% in adalimumab vs.46.7% in placebo -
16.7% endoscopic remission in the itolizumab arm at 12 weeks vs.16.7% in adalimumab vs.6.7% in placebo - Itolizumab was generally well tolerated, and no safety signal was observed
* One patient from the adalimumab arm had ‘endoscopic remission,’ but was recorded as ‘not in clinical remission’ at week 12 due to missing sub-score data
About Itolizumab
Itolizumab is a clinical-stage, first-in-class immune-modifying monoclonal antibody that selectively targets the CD6-ALCAM signaling pathway to downregulate pathogenic T effector cells while preserving T regulatory cells critical for maintaining a balanced immune response. This pathway plays a central role in modulating the activity and trafficking of T cells that drive a number of immuno-inflammatory diseases.
The blockade of CD6 with itolizumab has demonstrated a reduction in T effector cell proliferation and downregulation of several important pathways that contribute to T effector cell development. The downregulation of these pathways is accompanied by decreased secretion of the pro-inflammatory T effector cytokines IFN-γ, TNF-α, IL-6 and IL-17. Additionally, inhibiting the binding of ALCAM to CD6 modulates lymphocyte trafficking and results in reduced T effector cell infiltration into inflamed tissues.
About Biocon Limited
Biocon Limited, publicly listed in 2004, (BSE code: 532523, NSE Id: BIOCON, ISIN Id: INE376G01013) is an innovation-led global biopharmaceuticals company committed to enhance affordable access to complex therapies for chronic conditions like diabetes, cancer and autoimmune. It has developed and commercialized novel biologics, biosimilars, and complex small molecule APIs in
About Equillium
Equillium is a clinical-stage biotechnology company leveraging a deep understanding of immunobiology to develop novel therapeutics to treat severe autoimmune and inflammatory disorders with high unmet medical need. The company’s pipeline consists of the following novel first-in-class immunomodulatory assets and product platform targeting immuno-inflammatory pathways. Itolizumab: a monoclonal antibody that targets the CD6-ALCAM signaling pathway which plays a central role in the modulation of effector T cells that drive a number of immuno-inflammatory diseases. It is currently under evaluation in a Phase 3 clinical study of patients with acute graft-versus-host disease (aGVHD) and has exhibited positive data from both a Phase 2 clinical study of patients with moderate to severe ulcerative colitis and a Phase 1b clinical study of patients with lupus/lupus nephritis. Equillium acquired rights to itolizumab through an exclusive partnership with Biocon Limited, who also provides commercial manufacturing for the product. EQ101: a selective tri-specific cytokine inhibitor targeting IL-2, IL-9, and IL-15, has exhibited positive results in both a Phase 2 proof-of-concept clinical study of patients with moderate to severe alopecia areata and a Phase 1/2 proof-of-concept clinical study of patients with cutaneous T cell lymphoma (CTCL). EQ302: an orally delivered, selective bi-specific cytokine inhibitor targeting IL-15 and IL-21 at pre-clinical stage.
For more information, visit www.equilliumbio.com.
Forward-Looking Statements
Equillium
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words such as “anticipate”, “believe”, “could”, “continue”, “expect”, “estimate”, “may”, “plan”, “outlook”, “future”, “potential” and “project” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These statements include, but are not limited to, statements regarding Equillium’s plans and strategies with respect to developing itolizumab, the encouraging impact of the positive data in the context of Equillium’s Phase 3 EQUATOR study in aGVHD, the expected timeline for the presentation of additional data from the Phase 2 study of itolizumab in UC, and the potential benefits of Equillium’s product candidates. Because such statements are subject to risks and uncertainties, many of which are outside of Equillium’s control, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: Equillium’s ability to execute its plans and strategies; risks related to performing clinical and pre-clinical studies; whether the results from clinical and pre-clinical studies will validate and support the safety and efficacy of Equillium’s product candidates; changes in the competitive landscape; and changes in Equillium’s strategic plans. These and other risks and uncertainties are described more fully under the caption "Risk Factors" and elsewhere in Equillium's filings and reports, which may be accessed for free by visiting the Securities and Exchange Commission’s website and on Equillium’s website under the heading “Investors.” Investors should take such risks into account and should not rely on forward-looking statements when making investment decisions. All forward-looking statements contained in this press release speak only as of the date on which they were made. Equillium undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Biocon
This press release may include statements of future expectations and other forward-looking statements based on management's current expectations and beliefs concerning future developments and their potential effects upon Biocon and its subsidiaries/ associates. These forward-looking statements involve known or unknown risks and uncertainties that could cause actual results, performance or events to differ materially from those expressed or implied in such statements. Important factors that could cause actual results to differ materially from our expectations include, amongst other: general economic and business conditions in
View source version on businesswire.com: https://www.businesswire.com/news/home/20250206241338/en/
Corporate Contact
Michael Moore
Vice President, Investor Relations & Corporate Communications
619-302-4431
ir@equilliumbio.com
Biocon Limited
Saurabh Paliwal
Head – Investor Relations
+91 9538380801
saurabh.paliwal@biocon.com
Biocon Limited
Calvin Printer
Head – Corporate Communications
+91 7032969537
Calvin.printer@biocon.com
Source: Equillium, Inc.







