Dexcom G7 15 Day Receives FDA Clearance: the Longest Lasting Wearable and Most Accurate CGM System
- Dexcom G7 15 Day* is now cleared in the US for people age 18 years and above with diabetes.
- Now the longest lasting*,†,1 and most accurate1 CGM system, Dexcom G7 15 Day gives users the knowledge to better control diabetes.
- For people between the ages 2 to 18, Dexcom G7 remains the most accurate2 continuous glucose monitoring system.
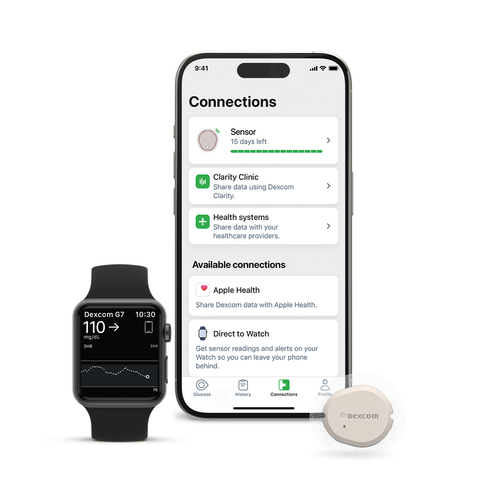
Dexcom G7 15 Day is now cleared in the US for people age 18 years and above with diabetes.
“The approval of Dexcom G7 15 Day marks another major innovation for Dexcom,” said Jake Leach, executive vice president and chief operating officer at Dexcom. “By listening to the needs of our users, we’re proud to offer the longest lasting wearable and most accurate CGM, giving people the knowledge to better control their diabetes with our best-in-class technology. This milestone sets a new standard in CGM and is a testament to our continued leadership in glucose biosensing. We look forward to bringing it to market in the second half of this year, but in the meantime, we encourage our users to upgrade to our current G7 system to gain the benefits of the most connected CGM brand in the world.”
New with Dexcom G7 15 Day
- Longest lasting*,†,1 CGM system with 15.5 days of wear.*
-
Best-in-class accuracy1 with an overall MARD of
8.0% .1,3 - Easier glucose management with fewer monthly sensors and reduced monthly waste.
Dexcom G7 features included with Dexcom G7 15 Day
- The only waterproof§ CGM available, keeping you more connected.
- Manage diabetes, hands-free. Connect your sensor directly to your Apple Watch|| so you can leave your iPhone behind and still see your glucose numbers.
- Automated activity logging,# simplified meal logging and new medication logging# help you better understand how activity, food and medications impact your glucose in real time.
- 12-hour grace period to replace finished sensors for a more seamless transition between sessions.
- Innovative and simple mobile app with Dexcom Clarity integration to easily view glucose patterns, trends and statistics via a range of interactive reports.**,††
- Ability to remotely share glucose numbers with caregivers and loved ones for added support and peace of mind.‡‡,9
- Enhanced and customizable alert settings for improved discretion.
“Dexcom G7 15 Day makes managing diabetes even easier with its extended wear and greater accuracy,” said Satish Garg, MD, from Barbara David Center for Diabetes at the University of Colorado School of Medicine. “Data recently released during the 18th international Advanced Technologies and Treatments for Diabetes conference in
Dexcom is working closely with its insulin pump partners to ensure that Dexcom G7 15 Day will be compatible with automated insulin delivery systems upon launch. Dexcom G7 15 Day is expected to launch in the US in the second half of 2025.
Visit Dexcom.com/start to get started with Dexcom G7 today. To learn more about Dexcom G7 15 Day and for additional information about when it will be available in the US, visit Dexcom.com/15day.
About Dexcom
Dexcom empowers people to take control of health through innovative biosensing technology. Founded in 1999, Dexcom has pioneered and set the standard in glucose biosensing for more than 25 years. Its technology has transformed how people manage diabetes and track their glucose, helping them feel more in control and live more confidently.
Dexcom. Discover what you’re made of. For more information, visit www.dexcom.com.
Category: IR
____________________ |
* A study was conducted to assess the sensor life where
1 Dexcom, Data on File, 2025. 2 Dexcom, Data on File, 2023. 3 Garg SK, et al. Diabetes Technol Ther. 2025. doi: 10.1089/dia.2025.0139. Epub ahead of print. 4 Beck RW, et al. JAMA. 2017;317(4):371-378. 5 Beck RW, et al. Ann Intern Med. 2017;167(6):365-374. 6 Martens T, et al. JAMA. 2021;325(22):2262-2272. 7 Laffel LM, et al. JAMA. 2020;323(23):2388-2396. 8 Welsh JB, et al. J Diabetes Sci Technol. 2024;18(1):143-7. 9 Polonsky WH, et al. Diabetes Technol Ther. 2021;23(3):195-202.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250410304660/en/
Media Contact
Nadia Conard
mediarelations@dexcom.com
Investor Contact
Sean Christensen
sean.christensen@dexcom.com
Source: DexCom, Inc.







