Delcath Systems Announces a Comparative Study of Chemosat and SIRT Published in the Journal Cancers
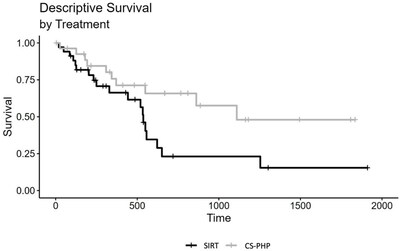
- Patients treated with Chemosat had significantly longer overall survival compared to those treated with SIRT (17 months vs. 9.9 months, respectively; p=0.006).
- None.
Insights
Analyzing...
Retrospective Comparison of Two Liver Directed Therapies in Patients with Metastatic Uveal Melanoma
Significantly Longer Overall Survival for Patients Treated with Chemosat vs. SIRT (17 and 9.9 months, respectively; p=0.006)
Uveal melanoma usually shows a liver-dominant metastasis spread and is often treated with liver directed therapies. This retrospective study compared two cohorts of patients with liver dominant uveal melanoma treated at the University Hospitals Tubingen,
Patients included in the study were treated between December 2013 and February 2020. Allocation to treatment was determined by a multidisciplinary team.
Presence of extrahepatic disease at baseline favored the SIRT group (
Disease control rates (DCR) were
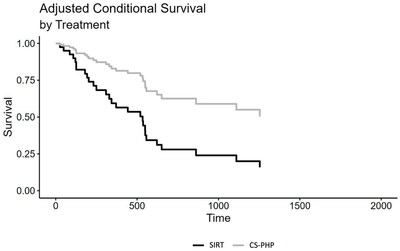
An adjusted Cox regression analysis, including the variables age, sex, presence of extrahepatic metastasis and hepatic load at baseline, also showed a significant effect of choice of treatment, HR = 0.32, CI
Median progression-free survival (mPFS) was 408.5 days for CS-PHP and 127.5 days for SIRT; the adjusted Cox regression analysis showed a trend favoring CS-PHP (p=0.090).
When initially presented at the 2023 CIRSE Annual Meeting held in
"There is a scarcity of comparative studies between liver directed therapies. This peer reviewed publication authored by experienced investigators, supports that the PHP procedure, whether utilizing melphalan delivered by Delcath's CE marked Chemosat or the FDA approved HEPZATO KIT, may be the preferred liver directed treatment option for patients with liver-dominant metastatic uveal melanoma," said Dr. Vojo Vukovic, Delcath's Chief Medical Officer. "We remain committed to making this treatment option available to patients in the US by the end of this year."
The full article can be viewed by clicking here.
About Chemosat and HEPZATO KIT
CHEMOSAT Hepatic Delivery System for Melphalan percutaneous hepatic perfusion (PHP) is designated under the medical device regulation for use in
HEPZATO KIT (melphalan for Injection/Hepatic Delivery System), approved for use in
HEPZATO KIT is approved in
HEPZATO KIT Important Safety Information
Patients eligible for HEPZATO should NOT have any of the following medical conditions:
- Active intracranial metastases or brain lesions with a propensity to bleed
- Liver failure, portal hypertension, or known varices at risk for bleeding
- Surgery or medical treatment of the liver in the previous 4 weeks
- Active cardiac conditions including unstable or severe angina or myocardial infarction), worsening or new-onset congestive heart failure, significant arrhythmias, or severe valvular disease
- History of allergies or known hypersensitivity to melphalan or a component or material utilized within the HEPZATO KIT including natural rubber latex, heparin, and severe hypersensitivity to iodinated contrast not controlled by antihistamines and steroids
Most common adverse reactions or laboratory abnormalities occurring with HEPZATO treatment are thrombocytopenia, fatigue, anemia, nausea, musculoskeletal pain, leukopenia, abdominal pain, neutropenia, vomiting, increased alanine aminotransferase, prolonged activated partial thromboplastin time, increased alkaline phosphatase, increased aspartate aminotransferase and dyspnea.
Severe peri-procedural complications including hemorrhage, hepatocellular injury, and thromboembolic events may occur via hepatic intra-arterial administration of HEPZATO. HEPZATO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy called the HEPZATO KIT REMS. Myelosuppression with resulting severe infection, bleeding, or symptomatic anemia may occur with HEPZATO. Additional cycles of HEPZATO therapy will be delayed until blood counts have improved.
Please see the full Prescribing Information, including BOXED WARNING for the HEPZATO KIT.
About Delcath Systems, Inc.
Delcath Systems, Inc. is an interventional oncology company focused on the treatment of primary and metastatic liver cancers. The Company's proprietary products, HEPZATO KIT (melphalan for Injection/Hepatic Delivery System), approved for use in
Forward Looking Statements
The Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This press release contains forward-looking statements, which are subject to certain risks and uncertainties, that can cause actual results to differ materially from those described. The words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "target," "will," "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Factors that may cause such differences include, but are not limited to, uncertainties relating to: the Company's commercialization plans and its ability to successfully commercialize the HEPZATO KIT; the Company's successful management of the HEPZATO KIT supply chain, including securing adequate supply of critical components necessary to manufacture and assemble the HEPZATO KIT; successful FDA inspections of the facilities of the Company and those of its third-party suppliers/manufacturers; the Company's successful implementation and management of the HEPZATO KIT Risk Evaluation and Mitigation Strategy; the potential benefits of the HEPZATO KIT as a treatment for patients with primary and metastatic disease in the liver; the Company's ability to obtain reimbursement for the HEPZATO KIT; and the Company's ability to successfully enter into any necessary purchase and sale agreements with users of the HEPZATO KIT. For additional information about these factors, and others that may impact the Company, please see the Company's filings with the Securities and Exchange Commission, including those on Forms 10-K, 10-Q, and 8-K. However, new risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. Accordingly, you should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
Contact:
Investor Relations Contact:
Ben Shamsian
Lytham Partners
646-829-9701
shamsian@lythampartners.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/delcath-systems-announces-a-comparative-study-of-chemosat-and-sirt-published-in-the-journal-cancers-301957002.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/delcath-systems-announces-a-comparative-study-of-chemosat-and-sirt-published-in-the-journal-cancers-301957002.html
SOURCE Delcath Systems, Inc.