Cybin Receives FDA Breakthrough Therapy Designation for its Novel Psychedelic Molecule CYB003 and Announces Positive Four-Month Durability Data in Major Depressive Disorder
- Breakthrough Therapy Designation granted by the FDA for CYB003 in the treatment of Major Depressive Disorder.
- Robust and sustained improvement in depression symptoms at four months with 75% remission after two 16mg doses.
- Mean 22-point reduction in MADRS score from baseline at four months.
- Data supports progression to a Phase 3 multinational study of CYB003 in MDD in mid-2024.
- CYB003 development program milestones achieved to expedite and de-risk the program.
- Positive topline results from Phase 2 trial support the Breakthrough Therapy Designation.
- CYB003 offers a potential treatment option for MDD patients who do not respond to existing therapies.
- Significant unmet medical need in depression highlighted by the BTD for CYB003.
- Conference call and webcast scheduled to discuss CYB003 program updates.
- None.
Insights
The Breakthrough Therapy Designation (BTD) granted by the FDA to CYB003 for Major Depressive Disorder (MDD) represents a significant milestone in the field of psychopharmacology. This designation is typically reserved for therapies that not only target serious conditions but also show substantial improvement over existing treatments. In the case of CYB003, a deuterated psilocybin analog, the robust and sustained efficacy demonstrated in Phase 2 trials indicates a potential paradigm shift in MDD treatment, particularly noteworthy given the reported remission rates and the magnitude of symptom improvement as measured by the Montgomery-Asberg Depression Rating Scale (MADRS).
From a medical research perspective, the BTD could significantly expedite CYB003's development timeline, enhancing the speed at which this treatment could become available to patients. This is of particular importance given the limitations of current antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), which leave a substantial portion of patients without adequate relief. The impact of CYB003 on remission rates and the potential for intermittent dosing present a compelling case for its continued development and the urgency for alternative MDD treatments.
The FDA's BTD for Cybin Inc.'s CYB003 has immediate financial implications for the company and its investors. This expedited pathway not only de-risks the development program by providing more intensive FDA guidance but also potentially shortens the time to market. This can be seen as a positive signal to investors, as the designation often leads to increased investor confidence and can positively influence the company's stock valuation. The anticipated Phase 3 multinational study and the potential for CYB003 to be the first FDA-approved adjunctive psychedelic-based therapy for MDD could represent a significant market opportunity, given the widespread prevalence of the disorder and the inadequacy of current treatments.
Furthermore, the favorable data points, such as the 75% remission rate and the impressive reduction in MADRS scores, suggest a strong efficacy profile that could translate into commercial success. Investors will be closely monitoring the progression of CYB003 through the pivotal trials and any strategic partnerships or funding opportunities that may arise as a result of this designation.
With the BTD for CYB003, Cybin Inc. positions itself at the forefront of an emerging market in psychedelic-based therapies. The positive reception of the BTD within the medical community, as evidenced by the commentary from Dr. Maurizio Fava, underscores a growing recognition of the potential for psychedelics in mental health treatment. This shift in perception, combined with the compelling clinical data, suggests a readiness in the market for innovative treatments like CYB003.
Given the high unmet need in MDD treatment, the market potential for CYB003 is considerable. The current landscape of antidepressant medications is crowded, yet many patients remain undertreated or suffer from treatment-resistant depression. CYB003's unique mechanism of action and dosing regimen could differentiate it in the competitive landscape. Market analysts will be keen to assess patient and clinician receptivity, payer coverage and the potential for CYB003 to capture market share from traditional antidepressants, as well as the impact on the broader mental health treatment market.
- Breakthrough Therapy Designation (“BTD”) provides an expedited review pathway, as well as increased access to
- First known BTD granted by the FDA for an adjunctive psychedelic based therapy for the treatment of Major Depressive Disorder (“MDD”) -
- Robust, sustained and statistically significant improvement in depression symptoms at four months with
- Impressive mean 22-point reduction in Montgomery-Asberg Depression Rating Scale (“MADRS”) score from baseline at four months -
- Data supports progression to a pivotal Phase 3 multinational study of CYB003 in MDD in mid-2024 -
- Achievement of milestones expedites and de-risks CYB003 development program -
- Company to host webcast to discuss CYB003 program updates today at 8:30 a.m. ET -
This news release constitutes a “designated news release” for the purposes of Cybin’s prospectus supplements each dated August 23, 2023, to its short form base shelf prospectus dated August 17, 2023, as amended December 22, 2023.
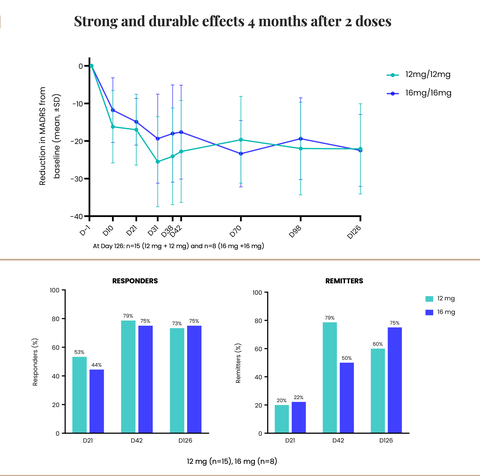
Strong and durable effects 4 months after 2 doses (Graphic: Business Wire)
The Company also announced that its Phase 2 trial of CYB003 in MDD demonstrated robust and sustained improvement in depression symptoms at four months with
These significant milestones reflect the Company’s commitment to advancing cutting-edge treatment options for MDD, marking a transformational moment in the pursuit of regulatory approval.
Breakthrough Therapy Designation Accelerates and De-risks the Path Forward6
BTD provides an expedited review pathway, as well as increased access to FDA guidance on trial design, with the potential to reduce drug development timelines. It is reserved for drug candidates that target serious conditions and demonstrate substantial improvement on a clinically significant endpoint over available therapies. The designation includes all “fast track” program features, as well as more intensive FDA guidance and discussion of the CYB003 development program, including planned clinical trials and plans for expediting the manufacturing development strategy.
The designation of CYB003 as a breakthrough therapy acknowledges the significant unmet medical need for more effective treatments of MDD and supports CYB003’s potential for significant improvements over existing therapies. BTD serves as validation of the Company’s progress to date and is expected to accelerate Cybin’s mission to advance its proprietary next-generation treatment towards new drug approval on an expedited basis.
This designation is supported by the positive topline results from the Company’s Phase 2 study of CYB003 in MDD, which demonstrated an improvement in depression symptoms superior to approved antidepressants and recently reported data with other psychedelics.1
“It is a testament to the hard work and dedication of the entire Cybin team that we have accomplished so much so quickly. The granting of Breakthrough Therapy Designation by the FDA underscores the potential of CYB003 to fill a gap in the treatment landscape for MDD and serves to expedite and de-risk our development program going forward,” stated Doug Drysdale, Chief Executive Officer of Cybin. “This designation provides for a streamlined review process and enhanced engagement with the FDA. With the robust durability data from our Phase 2 study in hand, we are ready to move forward expeditiously. We are grateful for the opportunity to accelerate the development and regulatory review process that this designation affords, as we prepare to advance CYB003 toward a Phase 3 pivotal trial around mid-year.”
“Currently available standard treatments for MDD can be limited in efficacy, remission and response rates, presenting challenges for patients and mental health practitioners alike. CYB003 may have potential to address these challenges, and with the FDA’s Breakthrough Therapy Designation, the regulatory path forward is accelerated,” said Dr. Maurizio Fava, M.D., Chair of the Department of Psychiatry and Psychiatrist-in-Chief at Massachusetts General Hospital.
Positive Four-Month Efficacy Data for CYB003
-
Robust and sustained improvements in symptoms of depression with two doses of 12 mg or 16 mg of CYB003:
- Mean reduction from baseline in the MADRS total score was approximately 22 points from baseline in both dosing cohorts.
-
Approximately
75% of the patients were responders (>/=50% improvement in MADRS scores) following two doses of 16mg. -
60% of patients on 12 mg and75% on 16 mg were in remission from depression following 2 doses (MADRS score </= 10).
Safety and tolerability:
- CYB003 was well tolerated with no drug-related serious adverse events.
- All adverse events were mild or moderate in intensity.
- No incidents of suicidal ideation or behavior.
- No discontinuations due to adverse events.
"The sustained reduction in depression symptoms at the four-month mark after just two doses of CYB003 is a critical milestone, that demonstrates the durability of the response, following the rapid improvement in symptoms. It also paves the way for a change in the treatment paradigm for MDD. Unlike currently approved adjunctive treatments which require chronic, daily dosing, CYB003 allows for intermittent dosing without the challenges of withdrawing patients from their existing medications,” stated Amir Inamdar, MBBS, DNB (Psych), MFPM, Chief Medical Officer of Cybin. “Notably, the durability data showed that at four months, approximately
“It is truly remarkable that at four months the participants experienced a sustained reduction and incremental improvement in depression symptoms,” continued Drysdale. “Impressively, the mean reduction from baseline in the MADRS total score was approximately 22 points at 4 months (compared to a mean reduction of 14 points vs placebo and 17 points from baseline at 3 weeks). This is highly encouraging, especially for patients who have not responded to existing treatment options. We look forward to initiating our Phase 3 trial, which we anticipate will be an international, multisite study to further evaluate the safety and efficacy of CYB003 capsules in a larger MDD patient population. As we advance this program, we are proud to lead the way and contribute to the growing body of scientific evidence supporting the therapeutic potential of psychedelic drugs to treat a multitude of mental health disorders,” concluded Drysdale.
The MADRS is a 10-item, clinician-administered scale designed to measure overall severity of depressive symptoms in subjects with MDD. It is widely used in clinical trials and accepted by regulatory authorities worldwide as a measure of symptoms of depression. The MADRS includes items ranging from sadness of mood, reduction in sleep and appetite, to difficulties in concentration, anhedonia, and negative and suicidal thoughts that are scored from 0 to 6, giving a total score ranging from 0 to 60. Typical score ranges for severity are: 0-6 normal; 7-19 mild; 20-34 moderate; and >34 severe depression. In the CYB003 study, mean baseline total scores on the MADRS were 31.4 to 33.7 in the active group and 30.8 in the placebo group.
Significant Unmet Medical Need in Depression
Depression is the leading cause of disability due to mental illness 2 and affects over 300 million people worldwide.3 Despite the use of currently available treatments such as selective serotonin reuptake inhibitors (“SSRIs”), up to two-thirds of patients with depression do not achieve remission with initial antidepressant treatment.4 Over 43 million Americans take antidepressants and over
Conference Call and Webcast Details:
Date: Wednesday, March 13, 2024
Time: 8:30 a.m. ET.
Dial-in: 800-267-6316 (
Conference ID: CYBN0313
Webcast: Register for the webcast here
The archived webcast will also be available on the Company’s investor relations website on the Events & Presentations page.
Notes:
- Stone et al. (2022) Response to acute monotherapy for major depressive disorder in randomized, placebo-controlled trials submitted to the US Food and Drug Administration: individual participant data analysis. BMJ (Clinical research ed.), 378, e067606.
- GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396: 1204–22.
- Friedrich M. J. (2017). Depression Is the Leading Cause of Disability Around the World. JAMA, 317(15), 1517. https://doi.org/10.1001/jama.2017.3826.
- Rush et al. Am J Psychiatry 2006; 163:1905–1917.
- Sood et al. (2023). Selective serotonin reuptake inhibitor use, age-related neuropathology and cognition in late-life. Psychiatry Research 328.
- There is no assurance that timelines will be met. Anticipated timelines regarding drug development are based on reasonable assumptions informed by current knowledge and information available to the Company. Such statements are informed by, among other things, regulatory guidelines for developing a drug with safety studies, proof of concept studies, and pivotal studies for new drug application submission and approval, and assume the success of implementation and results of such studies on timelines indicated as possible by such guidelines, other industry examples, and the Company’s development efforts to date.
About Cybin
Cybin is a clinical-stage biopharmaceutical company on a mission to create safe and effective psychedelic-based therapeutics to address the large unmet need for new and innovative treatment options for people who suffer from mental health conditions.
Cybin’s goal of revolutionizing mental healthcare is supported by a network of world-class partners and internationally recognized scientists aimed at progressing proprietary drug discovery platforms, innovative drug delivery systems, and novel formulation approaches and treatment regimens. The Company is currently developing CYB003, a proprietary deuterated psilocybin analog for the treatment of MDD and CYB004, a proprietary deuterated DMT molecule for generalized anxiety disorder and has a research pipeline of investigational psychedelic-based compounds.
Headquartered in
Cautionary Notes and Forward-Looking Statements
Certain statements in this news release relating to the Company are forward-looking statements and are prospective in nature. Forward-looking statements are not based on historical facts, but rather on current expectations and projections about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. These statements generally can be identified by the use of forward-looking words such as “may”, “should”, “could”, “intend”, “estimate”, “plan”, “anticipate”, “expect”, “believe” or “continue”, or the negative thereof or similar variations. Forward-looking statements in this news release include statements regarding the Company’s planned clinical trials and plans for expediting manufacturing and development strategy for CYB003; the potential for CYB003 to provide significant improvement over existing therapies; the advancement of CYB003 toward a Phase 3 trial in mid-2024; the potential reduction in drug development timelines afforded by BTD; and the Company’s plans to engineer proprietary drug discovery platforms, innovative drug delivery systems, novel formulation approaches and treatment regimens for mental health conditions.
These forward-looking statements are based on reasonable assumptions and estimates of management of the Company at the time such statements were made. Actual future results may differ materially as forward-looking statements involve known and unknown risks, uncertainties, and other factors which may cause the actual results, performance, or achievements of the Company to materially differ from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors, among other things, include: implications of the spread of COVID-19 on the Company's operations; fluctuations in general macroeconomic conditions; fluctuations in securities markets; expectations regarding the size of the psychedelics market; the ability of the Company to successfully achieve its business objectives; plans for growth; political, social and environmental uncertainties; employee relations; the presence of laws and regulations that may impose restrictions in the markets where the Company operates; and the risk factors set out in each of the Company's management's discussion and analysis for the three and nine month periods ended December 31, 2023 year, and the Company’s annual information form for the year ended March 31, 2023, which are available under the Company's profile on www.sedarplus.com and with the
Cybin makes no medical, treatment or health benefit claims about Cybin’s proposed products. The FDA, Health Canada or other similar regulatory authorities have not evaluated claims regarding psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds. The efficacy of such products has not been confirmed by approved research. There is no assurance that the use of psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds can diagnose, treat, cure or prevent any disease or condition. Rigorous scientific research and clinical trials are needed. Cybin has not conducted clinical trials for the use of its proposed products. Any references to quality, consistency, efficacy and safety of potential products do not imply that Cybin verified such in clinical trials or that Cybin will complete such trials. If Cybin cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on Cybin’s performance and operations.
Neither the Cboe Canada nor the NYSE American LLC stock exchange have approved or disapproved the contents of this news release and are not responsible for the adequacy and accuracy of the contents herein.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240313731043/en/
Investor & Media:
Gabriel Fahel
Chief Legal Officer
Cybin Inc.
1-866-292-4601
irteam@cybin.com – or – media@cybin.com
Source: Cybin Inc.







