Dr. Giovanni Selvaggi, Who Has Brought Several Oncology Drugs to Market, Joins CEL-SCI as Clinical Advisor
- Dr. Selvaggi was instrumental in the development and approval of lung cancer drugs Zykadia for Novartis and Opdivo for Bristol Myers Squibb
-
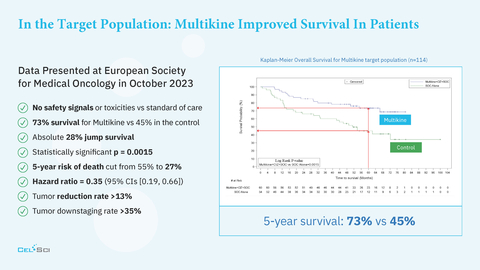
CEL-SCI’s Multikine immunotherapy improves the 5-year survival of head and neck cancer patients to
73% compared to45% in controls and cuts the 5-year risk of death by50% - FDA has given CEL-SCI the go-ahead to commence a confirmatory Registration Study for Multikine for the target population in head and neck cancer
- Dr. Selvaggi will be supporting CEL-SCI to bring Multikine to patients through a confirmatory registrational path that has been agreed with regulatory authorities and that has a potential for cure
(Graphic: Business Wire)
Multikine is the first investigational pre-surgical cancer drug in newly diagnosed head and neck cancer. Multikine is a copy of the pro-inflammatory cytokine immune response human bodies produce every day and is designed to empower a person’s intact immune system cells to attack their own cancer. Patients are treated with Multikine right after diagnosis, before any other standard of care treatment (surgery and radiation therapy) since that is when the immune system is strongest. There is robust safety and efficacy data from 750 patients who have been treated with Multikine.
Based on the following efficacy data, the FDA has agreed to CEL-SCI’s 212-person Registration Study to confirm the findings for regulatory approval:
- No safety signals vs standard of care
-
73% survival for Multikine vs45% in the control arm at 5 years - Statistically significant p = 0.0015
-
5-year risk of death cut in half from
55% to27% (Hazard ratio = 0.35 (95% CIs [0.19, 0.66]) - More detailed results may be viewed here: LINK
Dr. Selvaggi is a US-based drug developer, cancer researcher, and strategic advisor to big pharma and early-to-late stage biotech companies. He is currently Chief Medical Officer at Xcovery Holdings, where he manages an ongoing New Drug Application with the FDA for ensartinib, an ALK-TKI for non-small cell lung cancer (NSCLC). He is also a Clinical Consultant to Tubulis GmBH for a first-in-class ADC program in solid tumors, and Clinical Strategy Senior Advisor to Alira Health. He formerly served as Medical Director, Cancer Immunotherapy, in the MAGE-A3 lung cancer vaccine program at GSK. Later, Dr. Selvaggi played an instrumental role in the successful development and approval of ceritinib (Zykadia) in ALK-translocated NSCLC at Novartis. At Oncolytics he was VP of Clinical Development. Most recently, Dr. Selvaggi was part of the immunotherapy team at Bristol-Myers Squibb, serving as a program lead in thoracic malignancies, with a focus on SCLC and mesothelioma, leading to the approval of nivolumab (Opdivo) in third line small cell lung cancer. Dr. Selvaggi received his medical degree at the University of Torino School of Medicine, in
Dr. Selvaggi commented, “Multikine is a first in class immunotherapy that has demonstrated to meaningfully and safely prolong survival in a selected orphan population of newly diagnosed head and neck cancer. The importance of tackling cancer at the earliest stages to guarantee the most relevant impact in patients’ lives is undeniable, especially in head and neck cancer which has no approved therapeutic options before surgery. I am fully committed to supporting CEL-SCI leadership to bring this asset to patients through an expedited registrational path that has been agreed with regulatory authorities and that has a potential for cure.”
“Dr. Selvaggi has a passion for bringing cancer drugs to market to save lives and lead clinical research toward a cure for cancer. We are excited to have him join CEL-SCI as a Clinical Advisor at this pivotal point for Multikine which is set to commence a confirmatory FDA Registration Study,” stated CEL-SCI CEO, Geert Kersten. “The data on Multikine is excellent, and as we proceed with and wrap up this final study, Dr. Selvaggi’s experience and successful track record in navigating late-stage development, clinical trials, and the regulatory approval process will be hugely valuable for us.”
Dr. Selvaggi joins several other top-tier physician consultants and head and neck cancer key opinion leaders who are advisors to CEL-SCI.
About CEL-SCI Corporation
CEL-SCI believes that boosting a patient’s immune system while it is still intact should provide the greatest possible impact on survival. Multikine is designed to help the immune system "target" the tumor at a time when the immune system is still relatively intact and thereby thought to be better able to mount an attack on the tumor.
Multikine (Leukocyte Interleukin, Injection), a true first-line cancer therapy, has been dosed in over 750 patients and received Orphan Drug designation from the FDA for neoadjuvant therapy in patients with squamous cell carcinoma (cancer) of the head and neck. Multikine significantly extended life in its target patient population demonstrating a
The Company has operations in
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. When used in this press release, the words "intends," "believes," "anticipated," "plans" and "expects," and similar expressions, are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Such statements include, but are not limited to, statements about the terms, expected proceeds, use of proceeds and closing of the offering. Factors that could cause or contribute to such differences include an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI's filings with the Securities and Exchange Commission, including but not limited to its report on Form 10-K for the year ended September 30, 2023. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
* Multikine (Leukocyte Interleukin, Injection) is the trademark that CEL-SCI has registered for this investigational therapy. This proprietary name is subject to FDA review in connection with the Company's future anticipated regulatory submission for approval. Multikine has not been licensed or approved for sale, barter or exchange by the FDA or any other regulatory agency. Similarly, its safety or efficacy has not been established for any use.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240606671425/en/
Gavin de Windt
CEL-SCI Corporation
(703) 506-9460
Source: CEL-SCI Corporation







