FDA Clears AviClear® as a Long-Term Treatment for Mild to Severe Acne
AviClear by Cutera® makes history with another first to market milestone proving to be safe and effective across all skin types with long lasting results
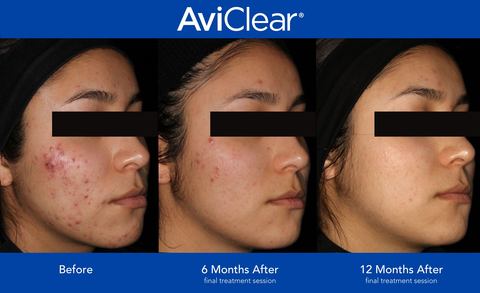
(Photo: Business Wire)
AviClear initially received FDA clearance in March 2022 following an extensive clinical trial. Now after months of clinical data evaluation, the FDA has additionally recognized AviClear as a clinically efficacious and proven treatment for the long-term treatment of acne. AviClear selectively targets and suppresses the sebaceous glands, eliminating acne at the source, offering a durable and prescription free option for patients and providers.
“Those of us who have been using AviClear on our patients since the initial FDA Clearance recognized that the results of the treatment get progressively better with time,” said Emmy M. Graber, MD, MBA, the Founder of The Dermatology Institute of
As the first 1726nm laser to be introduced to the market, AviClear continues to challenge the status quo in the acne landscape. In three, 30-minute treatment sessions
“We are proud to receive such a significant and landmark designation. The success of AviClear is a testament to Cutera’s ingenuity and innovation as a pioneering force in results-driven technology,” said Sheila A. Hopkins, Interim CEO at Cutera. “Throughout Cutera’s 25-year history, we have continued to develop devices that offer physicians and their patients breakthrough treatment options, and AviClear is a great example of our game changing technologies.”
Interested providers and patients are encouraged to visit www.AviClear.com for more information.
About Cutera, Inc.
1,2 Data on file. FDA clearance study. Cutera, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230615985076/en/
Media: EvolveMKD – Cutera@EvolveMKD.com
Investor Relations: Greg Barker, VP of FP & A – IR@Cutera.com
Source: Cutera, Inc.







