Cardiff Oncology Announces Positive Initial Data from First-line RAS-mutated mCRC Clinical Trial
Cardiff Oncology (NASDAQ: CRDF) announced positive initial data from its Phase 2 CRDF-004 trial evaluating onvansertib combined with standard-of-care (SoC) in first-line RAS-mutated metastatic colorectal cancer patients. The trial demonstrated a 64% objective response rate (ORR) in the 30mg onvansertib dose arm versus 33% ORR in the control arm.
The 30mg dose showed superior results compared to the 20mg dose (64% vs. 50% ORR) with deeper tumor regression. The drug was well-tolerated at both doses. The randomized trial enrolled patients with KRAS or NRAS mutations, combining onvansertib with FOLFIRI plus bevacizumab or FOLFOX plus bevacizumab. Additional clinical data is expected in 1H 2025.
Cardiff Oncology (NASDAQ: CRDF) ha annunciato dati iniziali positivi dal suo studio di Fase 2 CRDF-004 che valuta l'onvansertib combinato con il trattamento standard (SoC) nei pazienti con cancro colorettale metastatico RAS-mutato in prima linea. Lo studio ha dimostrato un tasso di risposta obiettivo (ORR) del 64% nel braccio con dose di 30mg di onvansertib rispetto al 33% di ORR nel braccio di controllo.
La dose di 30mg ha mostrato risultati superiori rispetto alla dose di 20mg (64% rispetto al 50% di ORR) con una regressione tumorale più profonda. Il farmaco è stato ben tollerato a entrambe le dosi. Lo studio randomizzato ha arruolato pazienti con mutazioni KRAS o NRAS, combinando onvansertib con FOLFIRI più bevacizumab o FOLFOX più bevacizumab. Ulteriori dati clinici sono attesi nel primo semestre del 2025.
Cardiff Oncology (NASDAQ: CRDF) anunció datos iniciales positivos de su ensayo de Fase 2 CRDF-004 que evalúa onvansertib combinado con el tratamiento estándar (SoC) en pacientes con cáncer colorrectal metastático mutado por RAS en primera línea. El ensayo demostró una tasa de respuesta objetiva (ORR) del 64% en el grupo de dosis de 30mg de onvansertib frente al 33% de ORR en el grupo de control.
La dosis de 30mg mostró resultados superiores en comparación con la dosis de 20mg (64% frente al 50% de ORR) con una regresión tumoral más profunda. El medicamento fue bien tolerado en ambas dosis. El ensayo aleatorizado inscribió a pacientes con mutaciones KRAS o NRAS, combinando onvansertib con FOLFIRI más bevacizumab o FOLFOX más bevacizumab. Se esperan datos clínicos adicionales en la primera mitad de 2025.
카디프 온콜로지 (NASDAQ: CRDF)는 RAS 변이 전이성 대장암 환자를 위한 1차 치료에서 표준 치료 (SoC)와 병합된 온반세르티브의 2상 CRDF-004 시험에서 긍정적인 초기 데이터를 발표했습니다. 이 시험에서 30mg 온반세르티브 투여군의 객관적 반응률 (ORR)은 64%로 나타났으며, 대조군은 33%의 ORR을 보였습니다.
30mg 용량은 20mg 용량(64% 대 50% ORR)보다 우수한 결과와 더 깊은 종양 퇴축을 보여주었습니다. 두 투여량 모두에서 약물은 잘 견딜 수 있었습니다. 이 무작위 시험은 KRAS 또는 NRAS 변이가 있는 환자를 등록하였으며, 온반세르티브와 FOLFIRI 및 베바시주맙 또는 FOLFOX 및 베바시주맙의 병합 요법을 시행했습니다. 추가 임상 데이터는 2025년 상반기에 예정되어 있습니다.
Cardiff Oncology (NASDAQ: CRDF) a annoncé des données initiales positives provenant de son essai de phase 2 CRDF-004 évaluant l'onvansertib combiné avec le traitement standard (SoC) chez des patients atteints d'un cancer colorectal métastatique muté par RAS en première ligne. L'essai a montré un taux de réponse objective (ORR) de 64% dans le bras dosé à 30mg d'onvansertib contre 33% d'ORR dans le bras de contrôle.
La dose de 30mg a montré des résultats supérieurs par rapport à la dose de 20mg (64% contre 50% d'ORR) avec une régression tumorale plus profonde. Le médicament a été bien toléré à обе doses. L'essai randomisé a recruté des patients avec des mutations KRAS ou NRAS, combinant l'onvansertib avec FOLFIRI plus bévacizumab ou FOLFOX plus bévacizumab. Des données cliniques supplémentaires sont attendues dans la première moitié de 2025.
Cardiff Oncology (NASDAQ: CRDF) gab positive erste Daten aus seiner Phase-2-Studie CRDF-004 bekannt, die onvansertib in Kombination mit der Standardbehandlung (SoC) bei Patienten mit RAS-mutiertem metastasiertem kolorektalem Krebs in der ersten Linie bewertet. Die Studie zeigte eine objektive Ansprechraten (ORR) von 64% in der Gruppe mit 30mg onvansertib im Vergleich zu 33% ORR in der Kontrollgruppe.
Die 30mg-Dosis zeigte überlegene Ergebnisse im Vergleich zur 20mg-Dosis (64% vs. 50% ORR) mit tieferer Tumorrückbildung. Das Medikament wurde bei beiden Dosen gut vertragen. Die randomisierte Studie rekrutierte Patienten mit KRAS- oder NRAS-Mutationen und kombinierte onvansertib mit FOLFIRI plus Bevacizumab oder FOLFOX plus Bevacizumab. Weitere klinische Daten werden im ersten Halbjahr 2025 erwartet.
- 64% ORR in 30mg dose arm vs 33% in control arm shows significant efficacy improvement
- Higher efficacy demonstrated in 30mg vs 20mg dose (64% vs 50% ORR)
- Well-tolerated safety profile at both doses
- Potential to address large market of 50,000 new US patients annually
- Small patient sample size (30 evaluable patients)
- Final data not expected until 1H 2025
Insights
The initial Phase 2 data for onvansertib shows remarkable promise in first-line RAS-mutated metastatic colorectal cancer treatment. The 64% objective response rate in the 30mg arm significantly outperforms both the control arm (33%) and the 20mg dose (50%). The dose-dependent efficacy, with deeper tumor regression at 30mg, suggests optimal therapeutic targeting of PLK1.
The dual compatibility with FOLFIRI and FOLFOX chemotherapy backbones is particularly significant, as it provides flexibility in treatment combinations - a key advantage over previous PLK1 inhibitors. With approximately 50,000 new RAS-mutated mCRC patients diagnosed annually in the US, the market potential is substantial if these results hold up in larger cohorts.
The favorable safety profile without major toxicities is important for first-line therapy acceptance. However, longer follow-up data on progression-free survival and duration of response will be critical for full clinical validation.
This clinical data represents a significant milestone for Cardiff Oncology, potentially expanding their addressable market in first-line mCRC treatment. The nearly doubled response rate in the 30mg arm compared to standard care could position onvansertib as a compelling first-line therapy option, particularly given its clean safety profile and compatibility with existing treatment regimens.
For a company with a market cap of
Investors should monitor duration of response data and progression-free survival metrics in future readouts, as these will be critical for market adoption and potential pricing power.
- Initial results from randomized Phase 2 CRDF-004 trial evaluating onvansertib + standard of care in RAS-mut mCRC demonstrated
- In the experimental arms, 30mg dose of onvansertib demonstrated a higher ORR compared to 20mg dose of onvansertib (
- Onvansertib was well tolerated at both doses -
- Additional clinical data from CRDF-004 trial expected in 1H 2025 -
- Company will hold a conference call today at 8:00 a.m. ET / 5:00 a.m. PT -
SAN DIEGO, Dec. 10, 2024 (GLOBE NEWSWIRE) -- Cardiff Oncology, Inc. (Nasdaq: CRDF), a clinical-stage biotechnology company leveraging PLK1 inhibition to develop novel therapies across a range of cancers, today announced positive initial data from CRDF-004, a randomized, Phase 2 clinical trial evaluating onvansertib in combination with standard-of-care (SoC) in patients with first-line RAS-mutated metastatic colorectal cancer (mCRC). Efficacy and safety data are for all evaluable patients as of a November 26, 2024 data cut-off date, and all efficacy data are determined by a blinded, independent central review (BICR) of each patient’s tumor scan.
“We are highly encouraged by the robust efficacy signal and favorable safety profile observed with onvansertib plus standard-of-care from the first 30 evaluable patients in our randomized first-line RAS-mutated mCRC CRDF-004 trial,” said Fairooz Kabbinavar, MD, FACP, Chief Medical Officer of Cardiff Oncology. “Our data shows an objective response rate of
Study Design
The CRDF-004 phase 2 trial is currently enrolling patients with mCRC who have a documented KRAS or NRAS mutation. Onvansertib is added to SoC consisting of FOLFIRI plus bevacizumab or FOLFOX plus bevacizumab. Patients are being randomized in a 1:1:1 ratio to either 20mg of onvansertib plus SoC, 30mg of onvansertib plus SoC, or SoC alone. The primary endpoint is objective response rate (ORR), and the secondary endpoints include progression-free survival (PFS), duration of response (DOR) and safety.
Efficacy Data
Objective Response Rates observed in the CRDF-004 clinical trial, as of the data cut-off date of November 26, 2024, are shown below.
| Control Arm (SoC alone) | 20mg dose of onvansertib + SoC | 30mg dose of onvansertib + SoC | All onvansertib patients |
(3 of 9) | (5 of 10) | (7 of 11) | (12 of 21) |
Spider Plots, displaying the change in tumor size from baseline for each patient over time, demonstrate deeper responses observed in patients receiving the 30mg dose of onvansertib in combination with the SoC compared to both the control arms and 20mg dose of onvansertib arms.
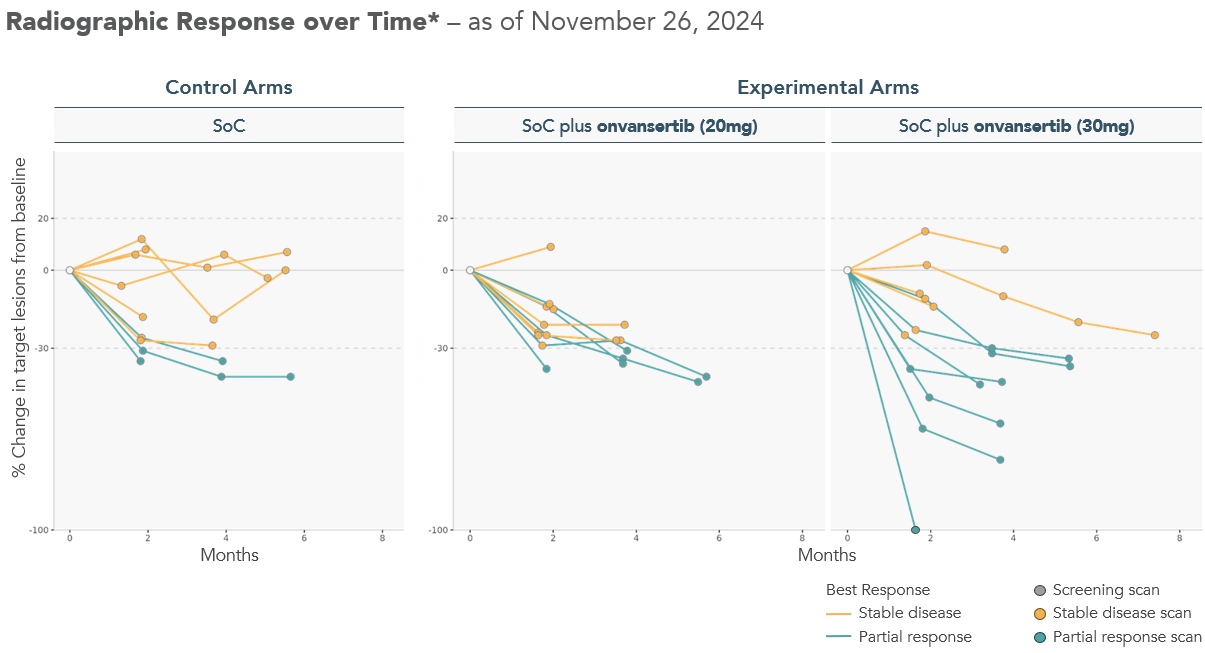
Note: Radiographic response determined per RECIST 1.1 by blinded independent central review. Spider plot reflects interim data as of November 26, 2024 from an ongoing trial and unlocked database.
Safety and Tolerability
Onvansertib in combination with chemo/bevacizumab was well-tolerated and there have been no major or unexpected toxicities observed.
“Overall, these data support our belief that onvansertib has potential to change the treatment paradigm for the entire first-line RAS-mutated mCRC patient population of almost 50,000 new patients diagnosed in the U.S. annually,” said Mark Erlander, Chief Executive Officer of Cardiff Oncology. “In addition to the efficacy signal observed, the data demonstrate that onvansertib can safely be combined with the two different chemo backbones that are currently approved as standard of care in the first-line setting, thus providing a key differentiated profile over previous generation PLK1 inhibitors. We look forward to providing additional clinical updates from our CRDF-004 trial in the first half of 2025.”
Upcoming expected milestones
- Additional clinical data from CRDF-004 trial expected in 1H 2025
Conference Call and Webcast
Cardiff Oncology will host a conference call and live webcast at 8:00 a.m. ET / 5:00 a.m. PT on December 10, 2024. Individuals interested in listening to the live conference call may do so by using the webcast link in the "Events" section of the company's website. A webcast replay will be available in the investor relations section on the company's website following the completion of the call.
About Cardiff Oncology, Inc.
Cardiff Oncology is a clinical-stage biotechnology company leveraging PLK1 inhibition, a well-validated oncology drug target, to develop novel therapies across a range of cancers. The Company's lead asset is onvansertib, a PLK1 inhibitor being evaluated in combination with standard of care (SoC) therapeutics in clinical programs targeting indications such as RAS-mutated metastatic colorectal cancer (mCRC), as well as in ongoing and planned investigator-initiated trials in metastatic pancreatic ductal adenocarcinoma (mPDAC), small cell lung cancer (SCLC) and triple negative breast cancer (TNBC). These programs and the Company's broader development strategy are designed to target tumor vulnerabilities in order to overcome treatment resistance and deliver superior clinical benefit compared to SoC alone. For more information, please visit https://www.cardiffoncology.com.
Forward-Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified using words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern Cardiff Oncology's expectations, strategy, plans or intentions. These forward-looking statements, including statements regarding Cardiff Oncology’s plans to provide additional clinical updates from our CRDF-004 trial in the first half of 2025, are based on Cardiff Oncology's current expectations and actual results could differ materially. There are several factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; our clinical trials may be suspended or discontinued due to unexpected side effects or other safety risks that could preclude approval of our product candidate; results of preclinical studies or clinical trials for our product candidate could be unfavorable or delayed; our need for additional financing; risks related to business interruptions, including the outbreak of an epidemic or pandemic such as the COVID-19 coronavirus and cyber-attacks on our information technology infrastructure, which could seriously harm our financial condition and increase our costs and expenses; uncertainties of government or third party payer reimbursement; dependence on key personnel; limited experience in marketing and sales; substantial competition; uncertainties of patent protection and litigation; dependence upon third parties; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. There are no guarantees that our product candidate will be utilized or prove to be commercially successful. Additionally, there are no guarantees that future clinical trials will be completed or successful or that our product candidate will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in Cardiff Oncology's Form 10-K for the year ended December 31, 2023, and other periodic reports filed with the Securities and Exchange Commission. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and Cardiff Oncology does not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances.
Cardiff Oncology Contact:
James Levine
Chief Financial Officer
858-952-7670
jlevine@cardiffoncology.com
Investor Contact:
Kiki Patel, PharmD
Gilmartin Group
332-895-3225
Kiki@gilmartinir.com
Media Contact:
Grace Spencer
Taft Communications
609-583-1151
A photo accompanying this announcement is available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/22880436-fc66-4f63-8937-5e69de0ce613








