CooperSurgical® Acquires AEGEA Medical
CooperSurgical has acquired California-based AEGEA Medical, known for its FDA-approved Mara™ Water Vapor Ablation System. This strategic acquisition enhances CooperSurgical's portfolio in women's healthcare, focusing on in-office treatments for heavy menstrual bleeding. The Mara system offers a two-minute, effective solution, demonstrated by clinical trials to improve quality of life for patients. With 99% of users reporting improved well-being, the acquisition aligns with CooperSurgical's commitment to innovative healthcare solutions.
- Acquisition of AEGEA Medical strengthens CooperSurgical's presence in women's healthcare.
- Mara Water Vapor Ablation System provides a quick, effective treatment option for heavy menstrual bleeding.
- Clinical trials show 99% of patients experienced quality of life improvements post-treatment.
- None.
Insights
Analyzing...
CooperSurgical announced today the acquisition of California-based, AEGEA Medical and its FDA-approved Mara™ Water Vapor Ablation System. The acquisition builds on over 30 years of experience in women’s healthcare and complements CooperSurgical’s growing portfolio of medical products focused on clinic and practice-based women’s health.
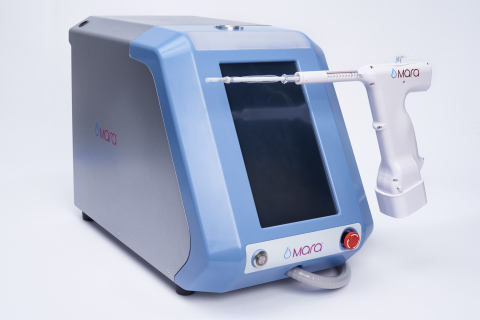
(Photo: Business Wire)
Mara is an endometrial ablation treatment that uses water vapor to safely treat heavy menstrual bleeding, specifically designed for use in the doctor’s office. Mara is a two-minute, in-office treatment that ablates the lining of the uterus, the source of heavy menstrual bleeding in women who have not reached menopause. Heavy menstrual bleeding, also known as menorrhagia, is a common condition about one-third of women seek treatment for during their lifetimes1.
Clinical study data demonstrates that Mara safely and effectively reduces heavy menstrual bleeding and improves a woman’s quality of life. Mara treats patients with myomas, large cavities and also provides post-ablation cavity access.
“I’d like to thank Maria Sainz and her team, CEO of AEGEA, for her dedication to innovation in women’s health,” said Holly Sheffield, President of CooperSurgical. “The Mara Water Vapor Ablation System gives women a great option for a safe and convenient in-office procedure to help with what can often be a debilitating health issue.”
Mara Clinical Results
One year after treatment in an international, multi-center pivotal clinical trial that enrolled 155 women,
-
99% of patients experienced improvement in quality of life3 -
91% of patients satisfied or very satisfied with procedure2,3 -
93% said they would recommend it to a friend2
For clinical information or a demo of Mara, please visit coopersurgical.com.
About CooperSurgical
For more than 30 years, CooperSurgical has been a leader in manufacturing and marketing a wide range of trusted and innovative brands that have assisted clinicians in advancing the standard of health care for women and families worldwide. CooperSurgical is a wholly-owned subsidiary of CooperCompanies. CooperSurgical, headquartered in Trumbull, CT, produces and markets a wide array of products and services for use by women’s health care clinicians. More information can be found at www.coopersurgical.com.
About CooperCompanies
CooperCompanies ("Cooper") is a global medical device company publicly traded on the NYSE (NYSE:COO). Cooper operates through two business units, CooperVision and CooperSurgical. CooperVision brings a refreshing perspective on vision care with a commitment to developing a wide range of high-quality products for contact lens wearers and providing focused practitioner support. CooperSurgical is committed to advancing the health of women, babies and families with its diversified portfolio of products and services focusing on medical devices and fertility & genomics. Headquartered in San Ramon, CA, Cooper has a workforce of more than 12,000 with products sold in over 100 countries. For more information, please visit www.coopercos.com.
###
References
- The American College of Obstetricians and Gynecologists. FAQ June 2016. https://www.acog.org/womens-health/faqs/heavy-menstrual-bleeding? AEGEA Medical, AEGEA Water Vapor System, SmartSeal, and IntegrityPro, and associated logos, are trademarks or registered trademarks of AEGEA Medical.
- AEGEA Medical Instructions For Use. https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160047D.pdf
- Levie MD, Chudnoff SG. A Prospective, Multicenter, Pivotal Trial to Evaluate the Safety and Effectiveness of the AEGEA Vapor Endometrial Ablation System. J Minim Invas Gynecol. May/June 2019; 26(4); 679-87
View source version on businesswire.com: https://www.businesswire.com/news/home/20210202005761/en/