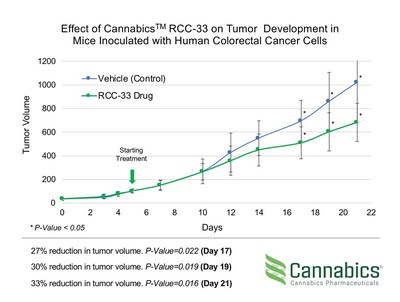
Cannabics Pharmaceuticals' in-vivo Study concludes with a 33% Lower Tumor Volume in Mice Treated with Company's Proprietary Drug Candidate for Colorectal Cancer
Cannabics Pharmaceuticals (OTCQB: CNBX) announced promising results from its in-vivo study on RCC-33 for colorectal cancer, showing a 33% reduction in tumor volume versus control (p ≤ 0.016). The study involved inoculated mice receiving daily doses of RCC-33, with significant differences noted after 10 days. The oncology therapeutics market is projected to reach $250 billion by 2024, with a focus on addressing a $10 billion colorectal cancer segment. Cannabics plans to prepare a product dossier for FDA submission and requests for a pre-IND meeting.
- 33% reduction in tumor volume in RCC-33 treated mice compared to control (p ≤ 0.016).
- Cannabics targets a $10 billion market in colorectal cancer with a significant unmet need.
- Plans for FDA submission and pre-IND meeting demonstrate progress in product development.
- None.
TEL AVIV, Israel and BETHESDA, Maryland, Feb. 16, 2021 /PRNewswire/ -- Cannabics Pharmaceuticals Inc. (OTCQB: CNBX), a global leader in the development of cancer related cannabinoid-based medicine, has just released the final results of its in-vivo study evaluating the efficacy of the company's proprietary drug candidate RCC-33 for the treatment of colorectal cancer in nude-mice. The final study results demonstrate a significant and robust inhibitory effect on tumor growth, as evidenced by a
Study design and results:
Both the experimental and the control groups were inoculated with human colorectal cancer cells. Daily doses of intraperitoneal (IP) delivery of RCC-33 or sham control were initiated on day 5. Differences in tumor volume between the two groups were first observed after 5 days of treatment (day 10). Previously published interim results showing a
Eyal Barad, Cannabics Pharmaceuticals' Co-founder and CEO said: "The outlook of the oncology market puts this sector's therapeutics sales forecast at
Gabriel Yariv, Cannabics Pharmaceuticals' President and COO said: "Having successfully completed this in-vivo POC study, the company is now planning to finalize its product dossier preparation for a forthcoming submission to the FDA along with a request for a pre-IND meeting for RCC-33. We are paving the way with a potentially new treatment approach for colorectal cancer patients, one that could possibly have an important positive impact on a large group of patients, and we feel comfortable and look forward to bringing our scientific data before the regulatory authorities for review".
About Cannabics Pharmaceuticals:
Cannabics Pharmaceuticals Inc. (OTCQB: CNBX) is a U.S. public company and a global leader in the development of cancer related cannabinoid-based medicine. The Company's R&D is based in Israel, where it is licensed by the Ministry of Health to conduct scientific and clinical research on cannabinoid formulations and cancer. For more information, please visit www.cannabics.com. For the latest updates on Cannabics Pharmaceuticals follow the Company on Twitter @Cannabics, Facebook @CannabicsPharmaceuticals, LinkedIn, and on Instagram @Cannabics_Pharmaceuticals.
Disclaimer:
Certain statements contained in this release may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other U.S. Federal securities laws. Such statements include but are not limited to statements identified by words such as "believes," "expects," "anticipates," "estimates," "intends," "plans," "targets," "projects" and similar expressions. The statements in this release are based upon the current beliefs and expectations of our Company's management and are subject to significant risks and uncertainties. Actual results may differ from those outlined in the forward-looking statements. Numerous factors could cause or contribute to such differences, including, but not limited to, results of clinical trials and other studies, the challenges inherent in new product development initiatives, the effect of any competitive products, our ability to license and protect our intellectual property, our ability to raise additional capital in the future that is necessary to maintain our business, changes in government policy and regulation, potential litigation by or against us, any governmental review of our products or practices, as well as other risks discussed from time to time in our filings with the Securities and Exchange Commission including, without limitation, our latest 10-Q Report filed January 14th, 2021. We undertake no duty to update any forward-looking statement or any information contained in this press release or other public disclosures at any time. Finally, the investing public is reminded that the only announcements or information about Cannabics Pharmaceuticals Inc., which are condoned by the Company, must emanate from the Company itself and bear our name as its source.
For more information about Cannabics:
Cannabics Pharmaceuticals Inc.
Phone: +1-(877)-424-2429
info@Cannabics.com
http://www.Cannabics.com
Related Links
https://www.cannabics.com
Photo - https://mma.prnewswire.com/media/1438876/Cannabics_RCC_33.jpg
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/cannabics-pharmaceuticals-in-vivo-study-concludes-with-a-33-lower-tumor-volume-in-mice-treated-with-companys-proprietary-drug-candidate-for-colorectal-cancer-301228846.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/cannabics-pharmaceuticals-in-vivo-study-concludes-with-a-33-lower-tumor-volume-in-mice-treated-with-companys-proprietary-drug-candidate-for-colorectal-cancer-301228846.html
SOURCE Cannabics Pharmaceuticals Inc.
FAQ
What were the results of Cannabics Pharmaceuticals' study on RCC-33 for colorectal cancer?
What is the market potential for Cannabics Pharmaceuticals' RCC-33?