CollPlant Announces Publication Highlighting its rhCollagen-based Photocurable Dermal Filler in the Plastic and Reconstructive Surgery Journal
Preclinical modeling demonstrates potential for filler to enhance cell proliferation and new tissue regeneration for aesthetic medicine applications
REHOVOT, Israel, Dec. 2, 2021 /PRNewswire/ -- CollPlant Biotechnologies (Nasdaq: CLGN), a regenerative and aesthetic medicine company developing innovative technologies and products for tissue regeneration and organ manufacturing, today announced the publication of an article in the Plastic and Reconstructive Surgery journal titled "The Potential Use of Novel Plant-Derived Recombinant Human Collagen in Aesthetic Medicine." The article highlights favorable in-vitro and in-vivo results of CollPlant's photocurable dermal filler as well as other potential applications of this collagen technology in aesthetic medicine.
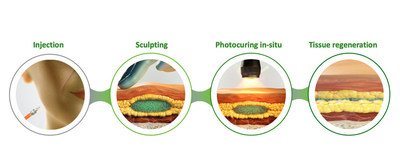
Following light illumination of the skin, CollPlant's photocurable dermal filler showed improved physical properties compared with standard of care, suggesting enhanced lifting effect and appearance. Biological properties assessed in a preclinical animal model, showed that the photocurable filler enhances cell proliferation and new tissue regeneration compared to standard of care.
"We are very pleased with these promising results of CollPlant's photocurable collagen technology, demonstrating its broad potential in aesthetic medicine applications", said Yehiel Tal, CollPlant's Chief Executive Officer. "Regenerative medicine aims to mimic nature, providing cells with growing environment as similar as possible to the original one. These published results further demonstrate recombinant human collagen's (rhCollagen) ability to serve as the ideal building block in regenerative medicine applications," Yehiel added.
CollPlant's photocurable dermal filler is composed of rhCollagen and hyaluronic acid and is intended for contour deficiencies corrections. The filler is designed to allow easy injection, followed by sculpting and in-situ hardening by light illumination of the skin.
The Plastic and Reconstructive Surgery journal is the official medical journal of the American Society of Plastic Surgeons, the world's largest organization of board-certified plastic surgeons representing more than 7,000 Member Surgeons.
About CollPlant
CollPlant is a regenerative and aesthetic medicine company focused on 3D bioprinting of tissues and organs, and medical aesthetics. The Company's products are based on its recombinant human collagen produced with CollPlant's proprietary plant based genetic engineering technology. These products address indications for the diverse fields of tissue repair, aesthetics, and organ manufacturing, and are ushering in a new era in regenerative and aesthetic medicine.
At the beginning of 2021, CollPlant entered into a development and global commercialization agreement for dermal and soft tissue fillers with Allergan, an AbbVie company, the global leader in the dermal filler market. Later in 2021, CollPlant entered into a strategic co-development agreement with 3D Systems for a 3D bioprinted regenerative soft tissue matrix for use in breast reconstruction procedures in combination with an implant.
For more information, visit http://www.collplant.com.
Safe Harbor Statements
This press release may include forward-looking statements. Forward-looking statements may include, but are not limited to, statements relating to CollPlant's objectives plans and strategies, as well as statements, other than historical facts, that address activities, events or developments that CollPlant intends, expects, projects, believes or anticipates will or may occur in the future. These statements are often characterized by terminology such as "believes," "hopes," "may," "anticipates," "should," "intends," "plans," "will," "expects," "estimates," "projects," "positioned," "strategy" and similar expressions and are based on assumptions and assessments made in light of management's experience and perception of historical trends, current conditions, expected future developments and other factors believed to be appropriate. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements. Many factors could cause CollPlant's actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including, but not limited to, the following: the Company's history of significant losses, its ability to continue as a going concern, and its need to raise additional capital and its inability to obtain additional capital on acceptable terms, or at all; the impact of the COVID-19 pandemic; the Company's expectations regarding the timing and cost of commencing clinical trials with respect to tissues and organs which are based on its rhCollagen based BioInk and products for medical aesthetics; the Company's ability to obtain favorable pre-clinical and clinical trial results; regulatory action with respect to rhCollagen based BioInk and medical aesthetics products including but not limited to acceptance of an application for marketing authorization review and approval of such application, and, if approved, the scope of the approved indication and labeling; commercial success and market acceptance of the Company's rhCollagen based products in 3D Bioprinting and medical aesthetics; the Company's ability to establish sales and marketing capabilities or enter into agreements with third parties and its reliance on third party distributors and resellers; the Company's ability to establish and maintain strategic partnerships and other corporate collaborations; the Company's reliance on third parties to conduct some or all aspects of its product manufacturing; the scope of protection the Company is able to establish and maintain for intellectual property rights and the Company's ability to operate its business without infringing the intellectual property rights of others; the overall global economic environment; the impact of competition and new technologies; general market, political, and economic conditions in the countries in which the Company operates; projected capital expenditures and liquidity; changes in the Company's strategy; and litigation and regulatory proceedings. More detailed information about the risks and uncertainties affecting CollPlant is contained under the heading "Risk Factors" included in CollPlant's most recent annual report on Form 20-F filed with the SEC, and in other filings that CollPlant has made and may make with the SEC in the future. The forward-looking statements contained in this press release are made as of the date of this press release and reflect CollPlant's current views with respect to future events, and CollPlant does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact at CollPlant:
Eran Rotem
Deputy CEO & Chief Financial Officer
Tel: + 972-73-2325600/631
Email: Eran@collplant.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/collplant-announces-publication-highlighting-its-rhcollagen-based-photocurable-dermal-filler-in-the-plastic-and-reconstructive-surgery-journal-301436107.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/collplant-announces-publication-highlighting-its-rhcollagen-based-photocurable-dermal-filler-in-the-plastic-and-reconstructive-surgery-journal-301436107.html
SOURCE CollPlant