LumiraDx, a Next-Generation Point of Care Diagnostics Testing Company to List on Nasdaq via Merger with CA Healthcare Acquisition Corp
CA Healthcare Acquisition Corp (Nasdaq: CAHC) has entered into a definitive merger agreement with LumiraDx Limited, valuing LumiraDx at $5.0 billion. LumiraDx, a leader in point of care diagnostics, has raised $700 million since its inception, with significant investments from notable partners. The company’s COVID-19 antigen test has received Emergency Use Authorization from the FDA and is deployed in numerous countries globally. A new financing of $400 million has been secured to support growth and product development. The deal is anticipated to close late Q2 or early Q3 2021, subject to shareholder approval.
- Merger with LumiraDx valued at $5.0 billion, enhancing CAHC's portfolio.
- Significant demand growth for LumiraDx's COVID-19 testing solutions.
- Successful financing of $400 million to bolster operations and R&D.
- Projected 2021 revenue for LumiraDx between $600 million and $1 billion.
- None.
CA Healthcare Acquisition Corp (Nasdaq: CAHC), a special purpose acquisition company focused on investing in a growth-oriented healthcare company which recently raised
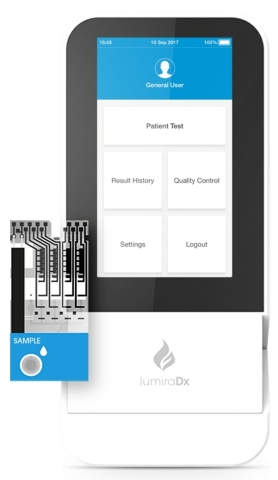
LumiraDx Platform and Test Strip (Photo: Business Wire)
LumiraDx has developed its high sensitivity antigen test for COVID-19 on the LumiraDx Platform. The test is currently being used by the National Health Service (NHS) and Boots in the UK, CVS Health in the U.S., a significant number of accident and emergency rooms in Italy and other parts of Europe and is being deployed in partnership with the Bill and Melinda Gates Foundation in a growing number of African countries where access to laboratory diagnostics is limited. The LumiraDx COVID-19 antigen test has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) and achieved CE Mark. The LumiraDx Platform and COVID-19 antigen tests are also available in Japan and Brazil and being rolled out in more than 60 countries globally.
The LumiraDx Platform menu also includes point of care tests for COVID-19 Antibody, INR and D-Dimer - with high levels of accuracy comparable to central lab-based tests – all of which have achieved CE Mark and are commercially available in Europe. The Platform is designed to go wherever the patient is, whether this is in a hospital, medical office, pharmacy, or in other non-traditional settings such as schools or airports.
“LumiraDx is at the tipping point of driving a transformation in diagnostic testing. This new public recognition will solidify our already growing presence in the point of care testing market,” said Ron Zwanziger, Chairman and CEO of LumiraDx. “COVID-19 has demonstrated how important it is to have rapid and highly accurate diagnostic tests, at mass scale, and available everywhere. It has validated the performance of our Platform and enabled us to partner with governments, health systems, retail chains and other customers to expand testing across community care settings both in high and low-and middle-income countries. This access to increased testing will change the way care pathways are currently practiced, improving patient outcomes and saving human lives.”
“LumiraDx’s next-generation point of care solutions provide a significant opportunity for our shareholders,” said Larry Neiterman, Chairman and CEO of CAHC. “Ron and his management team have decades of entrepreneurial success in innovative diagnostics businesses and the LumiraDx Platform and testing menu offer healthcare providers and other customers major advantages over traditional central labs. LumiraDx has a clear strategy for addressing the large and underpenetrated testing market to increase next-generation POC market share. In the near-term, demand for fast, low-cost COVID-19 tests is driving strong and transformational growth for LumiraDx’s solutions.”
Ron Zwanziger and his proven management team will continue to lead LumiraDx post-transaction. He and a core group of executives founded LumiraDx in 2014 after previously founding and growing a number of successful POC diagnostic companies that were then sold to global healthcare companies for an aggregate consideration of more than
LumiraDx – Next-Generation POC Diagnostics
LumiraDx is headquartered in the UK with R&D and manufacturing centers in Scotland, England and the U.S., and sales and marketing operations across Western Europe, the U.S., Japan, South Africa, Colombia and Brazil. The company has more than 1,200 employees across 17 countries.
The LumiraDx Platform simplifies, scales down, and integrates principles used in lab systems, to deliver accurate results compared to laboratory reference assays across a number of parameters, in a portable, easy-to-use point of care solution. The Platform has been designed to integrate the most commonly used assay technologies such as enzyme, immunoassay, molecular and electrolytes as well as sample types such as swab, saliva, and blood. The multi-channel, low-cost test strips allow for precise control and optimization of each test.
In addition to COVID-19, the Platform can perform tests that are commercially available or in development for other infectious disease, cardiovascular disease, coagulation disorders and diabetes. A number of regulatory submissions to expand the menu of available tests are planned or underway in the U.S., EU, UK, Japan as well as many other countries.
Later this year, subject to regulatory approval or clearance, LumiraDx also plans to launch Amira, a low-cost mass-screening and home testing system for COVID-19, which will support widespread efforts to safely reopen the economy. LumiraDx anticipates the retail price of Amira will be
LumiraDx estimates 2021 revenue of
LumiraDx also announced today that it has secured two new financing commitments totaling
Summary of Merger Transaction
The transaction implies an equity valuation at closing for the combined company of in excess of
The combined company will be led by existing CEO Ron Zwanziger and the other Co-Founders, and LumiraDx’s existing board and governance principles will not change. Upon closing of the transaction, LumiraDx and its common shares are expected to trade on Nasdaq under the ticker symbol "LMDX." The transaction is currently expected to close late Q2, early Q3 this year, subject to approval by the securityholders of each of CAHC and LumiraDx and satisfaction of customary closing conditions.
The transaction has been unanimously approved by the Board of Directors of both LumiraDx and CA Healthcare Acquisition Corp.
For a summary of the material terms of the proposed transaction, including a copy of the definitive agreement and investor presentation, please see the Current Report on Form 8-K to be filed today with the U.S. Securities and Exchange Commission (the “SEC”) by CAHC and available at www.sec.gov.
All materials may also be found at https://www.cahcspac.com/investor-relations
Advisors
Evercore, Inc. and Raymond James & Associates, Inc. are serving as financial advisors to LumiraDx. BTIG, LLC is serving as financial advisor and capital markets advisor to CA Healthcare Acquisition Corp. Fried, Frank, Harris, Shriver & Jacobson LLP and Goodwin Procter LLP are serving as legal advisors to LumiraDx. Sidley Austin LLP is serving as legal advisor to CA Healthcare Acquisition Corp.
Additional Information
About LumiraDx
LumiraDx was founded in 2014 by a group of entrepreneurs: Ron Zwanziger, our Chairman and Chief Executive Officer; Dave Scott, Ph.D., our Chief Technology Officer; and Jerry McAleer, Ph.D., our Chief Scientist, who have a successful track record in building and scaling diagnostics businesses over three decades, including at companies such as Medisense, Inc., Inverness Medical Technology Inc. and Alere Inc. The company is supported by institutional and strategic investors including the Bill & Melinda Gates Foundation, Morningside Ventures, U.S. Boston Capital Corporation, and Petrichor Healthcare Capital Management. Based in the UK and supported by its worldwide affiliates to provide access in all major markets, LumiraDx has more than 1,200 employees worldwide.
LumiraDx develops, manufactures and commercializes an innovative point of care diagnostic Platform. The LumiraDx Platform is designed to deliver lab comparable diagnostic results at the point of care in minutes. It is designed to be affordable and accessible for healthcare providers globally, and to strengthen community-based healthcare.
Further information on LumiraDx and the LumiraDx Platform is available at www.lumiradx.com
About CA Healthcare Acquisition Corp.
CA Healthcare Acquisition Corp. is a special purpose acquisition company formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. For more information, visit www.cahcspac.com/.
Forward-Looking Statements
Certain statements in this press release may be considered “forward-looking statements” within the meaning of the “safe harbor” provisions of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally relate to future events or CAHC’s or LumiraDx’s future financial or operating performance. For example, projections of future revenue, total addressable market and other metrics are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “expect,” “intend,” “will,” “estimate,” “anticipate,” “believe,” “predict” or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by CAHC and its management, and LumiraDx and its management, as the case may be, are inherently uncertain. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: 1) the occurrence of any event, change or other circumstances that could give rise to the termination of the definitive merger agreement with respect to the business combination; 2) the outcome of any legal proceedings that may be instituted against CAHC, the combined company or others following the announcement of the business combination and any definitive agreements with respect thereto; 3) the inability to complete the business combination due to the failure to obtain approval of the securityholders of CAHC or LumiraDx, to obtain financing to complete the business combination or to satisfy other conditions to closing; 4) changes to the proposed structure of the business combination that may be required or appropriate as a result of applicable laws or regulations or as a condition to obtaining regulatory approval of the business combination; 5) the ability to meet the Nasdaq’s listing standards following the consummation of the business combination; 6) the risk that the business combination disrupts current plans and operations of LumiraDx as a result of the announcement and consummation of the business combination; 7) the ability to recognize the anticipated benefits of the business combination, which may be affected by, among other things, competition, the ability of the combined company to grow and manage growth profitably, maintain relationships with customers, manufacturers and suppliers and retain its management and key employees; 8) costs related to the business combination; 9) changes in applicable laws or regulations; 10) the possibility that LumiraDx or the combined company may be adversely affected by other economic, business and/or competitive factors; 11) LumiraDx’s estimates of its financial performance; and 12) other risks and uncertainties set forth in the section entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in CAHC’s Registration Statement on form S-1 filed with the SEC on January 8, 2021 and the registration statement on Form F-4 and proxy statement/prospectus discussed below. Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Neither CAHC nor LumiraDx undertakes any duty to update these forward-looking statements, except as otherwise required by law.
Use of Projections
This press release contains financial forecasts of LumiraDx, namely LumiraDx’s projected revenue for 2021. Neither LumiraDx’s independent auditors, nor the independent registered public accounting firm of CAHC, audited, reviewed, compiled or performed any procedures with respect to the projections for the purpose of their inclusion in this press release, and accordingly, neither of them expressed an opinion or provided any other form of assurance with respect thereto for the purpose of this press release. These projections should not be relied upon as being necessarily indicative of future results. The projected financial information contained in this press release constitutes forward-looking information. The assumptions and estimates underlying such projected financial information are inherently uncertain and are subject to a wide variety of significant business, economic, competitive and other risks and uncertainties that could cause actual results to differ materially from those contained in the prospective financial information. See “Forward-Looking Statements” above. Actual results may differ materially from the results contemplated by the projected financial information contained in this press release, and the inclusion of such information in this press release should not be regarded as a representation by any person that the results reflected in such projections will be achieved.
Additional Information About the Proposed Business Combination and Where to Find It
In connection with the proposed business combination, LumiraDX intends to file with the SEC a registration statement on Form F-4 containing a preliminary proxy statement of CAHC and a preliminary prospectus of LumiraDx, and after the registration statement is declared effective, CAHC will mail a definitive proxy statement/prospectus relating to the proposed business combination to its shareholders. This press release does not contain all the information that should be considered concerning the proposed business combination and is not intended to form the basis of any investment decision or any other decision in respect of the business combination. CAHC’s shareholders and other interested persons are advised to read, when available, the preliminary proxy statement/prospectus and the amendments thereto and the definitive proxy statement/prospectus and other documents filed in connection with the proposed business combination, as these materials will contain important information about LumiraDx, CAHC and the proposed business combination. When available, the definitive proxy statement/prospectus and other relevant materials for the proposed business combination will be mailed to shareholders of CAHC as of a record date to be established for voting on the proposed business combination. Such shareholders will also be able to obtain copies of the preliminary proxy statement/prospectus, the definitive proxy statement/prospectus and other documents filed with the SEC, without charge, once available, at the SEC’s website at www.sec.gov, or by directing a request to CA Healthcare Acquisition Corp., 99 Summer Street, Suite 200 Boston, MA 02110.
This press release does not constitute an offer to sell or the solicitation of an offer to buy any securities, or a solicitation of any vote or approval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the U.S. Securities Act of 1933, as amended.
Participants in the Solicitation
CAHC and its directors and executive officers may be deemed participants in the solicitation of proxies from CAHC’s shareholders with respect to the proposed business combination. A list of the names of those directors and executive officers and a description of their interests in CAHC is contained in CAHC’s Registration Statement on form S-1 filed with the SEC on January 8, 2021, which is available free of charge at the SEC’s website at www.sec.gov. Additional information regarding the interests of such participants will be contained in the proxy statement/prospectus for the proposed business combination when available.
LumiraDx and its directors and executive officers may also be deemed to be participants in the solicitation of proxies from the shareholders of CAHC in connection with the proposed business combination. A list of the names of such directors and executive officers and information regarding their interests in the proposed business combination will be included in the proxy statement/prospectus for the proposed business combination when available.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210406006194/en/
FAQ
What is the merger value between CAHC and LumiraDx?
When is the CAHC and LumiraDx merger expected to close?
What financing has LumiraDx secured?
What is the projected revenue for LumiraDx in 2021?







