Boston Scientific Receives FDA Approval for FARAPULSE™ Pulsed Field Ablation System
- FDA approval for the FARAPULSE PFA System
- Positive 12-month data from the pivotal ADVENT clinical trial
- No reports of permanent phrenic nerve palsy, pulmonary vein stenosis or esophageal injury in real-world data
- Plans for immediate launch in the U.S.
- Potential competition from other ablation technologies in the market
- The need for regulatory approval for the navigation-enabled version of the FARAWAVE catheter
Insights
The approval of the FARAPULSE PFA System by the FDA signifies a considerable advancement in the field of electrophysiology, particularly for the treatment of paroxysmal atrial fibrillation (AF), a common and disruptive heart rhythm disorder. The system's non-thermal approach to ablation presents a significant innovation, as it selectively targets cardiac tissue while minimizing potential damage to adjacent structures—a concern with traditional thermal ablation techniques.
The clinical implications are noteworthy, with the potential to reduce procedural times and associated risks, thereby improving patient outcomes. The positive results from the ADVENT clinical trial, which suggest comparable safety and efficacy to conventional ablation, coupled with a faster learning curve for physicians, could make the FARAPULSE PFA System a preferred option in clinical practice, leading to broader adoption in the management of AF.
From a market perspective, the FDA approval of the FARAPULSE PFA System positions Boston Scientific at the forefront of the growing PFA space. The system's differentiation in terms of safety and efficiency could disrupt the current standard-of-care for AF ablation, potentially capturing a significant market share. This is underscored by the system's prior Breakthrough Device Designation and earlier CE Mark approval, indicating both regulatory support and a positive reception in the European market.
The immediate launch in the U.S. market and ongoing clinical trials for expanded indications, such as the ADVANTAGE AF and AVANT GUARD, suggest a strategic approach to cementing the system's clinical utility and market penetration. Investors may anticipate a positive impact on Boston Scientific's revenue growth, especially considering the system's potential to become a practice-changing technology in a high-prevalence condition like AF.
The trajectory of the FARAPULSE PFA System, from receiving Breakthrough Device Designation to FDA approval, demonstrates a successful regulatory strategy by Boston Scientific. This pathway is designed to expedite the development and review of devices that provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases. The system's approval is also a testament to the robustness of the clinical evidence base supporting its safety and efficacy.
Looking ahead, the anticipated regulatory approval for the navigation-enabled version of the FARAWAVE catheter and FARAVIEW Software Module in 2024 will be another critical milestone. The regulatory process for these adjunct technologies will likely scrutinize the integrated system's safety profile and effectiveness, further solidifying its clinical and commercial positioning.
Strong clinical evidence base, largest volume of real-world use reinforce safety, efficacy and efficiency advantages of the system
"The approval of the FARAPULSE PFA System marks an important milestone for the millions of people living with paroxysmal AF and is an incredible opportunity to bring the first PFA system designed and built solely for this type of ablation therapy to physicians in the
During a traditional ablation procedure, a catheter is guided to the interior of the heart and generates extreme temperatures – hot or cold – to destroy targeted areas associated with abnormal heart rhythms. The FARAPULSE PFA System, however, relies on tissue-selective, non-thermal electric fields to ablate heart tissue and avoid damage to surrounding structures. Positive 12-month data from the pivotal ADVENT clinical trial – the first randomized clinical trial to directly compare the efficacy and safety of the system against standard-of-care ablation – found that therapy with the device was as safe and effective as conventional thermal ablation, with statistically shorter ablation times and a quick learning curve for physicians. Additional real-world data from more than 17,000 patients in the MANIFEST-17K registry demonstrated continued real-world safety of the system, with no reports of permanent phrenic nerve palsy, pulmonary vein stenosis or esophageal injury.
"Within the ADVENT clinical trial, the FARAPULSE PFA System was shown to be a safe, effective and efficient option for treating paroxysmal AF, and extensive global real-world use has mirrored that profile," said Vivek Reddy, M.D., director of electrophysiology, Mount Sinai Fuster Heart Hospital,
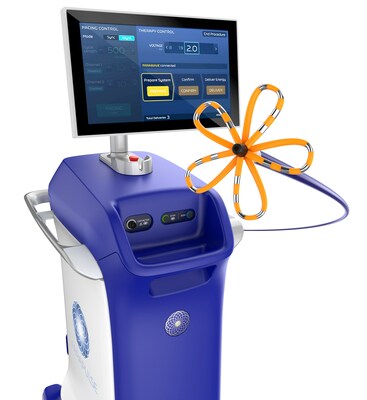
The FARAPULSE PFA System delivers pulsed field energy and consists of the FARAWAVE™ Ablation Catheter, the FARASTAR™ Ablation Generator, and the FARADRIVE™ Steerable Sheath, which is complemented by the VersaCross Connect™ Access Solution for the FARADRIVE Steerable Sheath to provide safe and efficient access to the left side of the heart during procedures with the system. The FARAWAVE catheter is used to treat a range of pulmonary vein anatomies using an over-the-wire catheter with variable basket and flower shapes, allowing the device to adapt to individual patient anatomies. These configurations reinforce ease-of-use for physicians and promote reproducible procedures between operators.
Boston Scientific completed enrollment in the first phase of the ADVANTAGE AF clinical trial in the third quarter of 2023, which is studying the system for the treatment of patients with drug-refractory, symptomatic, persistent AF, and commenced enrollment in a second phase of the study to evaluate the safety and effectiveness of adjunctive use of the FARAPOINT™ PFA Catheter for cavotricuspid isthmus (CTI) ablations, a procedure used to treat atrial flutter. The company also recently commenced the AVANT GUARD clinical trial to evaluate the safety and efficacy of the system as a first-line treatment for persistent AF compared to anti-arrhythmic drug therapy.
The FARAPULSE PFA System was granted Breakthrough Device Designation from the Center for Devices and Radiological Health (CDRH) of the
More information on the FARAPULSE PFA System is available here.
About Boston Scientific
Boston Scientific transforms lives through innovative medical technologies that improve the health of patients around the world. As a global medical technology leader for more than 40 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs and reduce the cost of healthcare. Our portfolio of devices and therapies helps physicians diagnose and treat complex cardiovascular, respiratory, digestive, oncological, neurological and urological diseases and conditions. Learn more at www.bostonscientific.com and connect on LinkedIn and X, formerly Twitter.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our business plans and product performance and impact, new and anticipated product approvals and launches, and clinical trials. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; manufacturing, distribution and supply chain disruptions and cost increases; new product introductions; expected procedural volumes; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Steve Bailey
Media Relations
(651) 582-4343 (office)
Steve.Bailey@bsci.com
Lauren Tengler
Investor Relations
(508) 683-4479
BSXInvestorRelations@bsci.com
*Dr. Vivek Reddy is a paid consultant of Boston Scientific Corporation. He has not been compensated in connection with this press release.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/boston-scientific-receives-fda-approval-for-farapulse-pulsed-field-ablation-system-302048807.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/boston-scientific-receives-fda-approval-for-farapulse-pulsed-field-ablation-system-302048807.html
SOURCE Boston Scientific Corporation